Phase 3 Trial of Experimental Gout Drug Lesinurad to Begin
Ardea Biosciences announced on Monday that it would soon begin the first of four planned Phase 3 clinical trials of its experimental gout drug lesinurad.
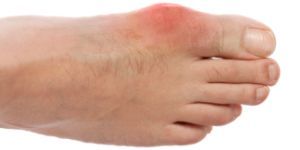
Ardea Biosciences announced on Monday that it would soon begin the first of four planned Phase 3 clinical trials of its experimental gout drug lesinurad. The trial is expected to include around 2,000 gout patients at study sites around the world.
Lesinurad is an oral inhibitor of the URAT1 transporter in the kidney that regulates uric acid excretion. According to a press release from Ardea Biosciences, around 90% of gout patients are thought to excrete too little uric acid, and studies have found that defective renal transporters, which promote excretion of uric acid under normal circumstances, are genetically linked to gout.
Since currently available gout drugs such as the xanthine oxidase inhibitors allopurinol and febuxostat have a mechanism of action different from URAT1 inhibitors, the hope is that lesinurad will be able to be used in conjunction with these other drugs to help the many gout patients who are not adequately treated with existing therapies alone. In previous Phase 1 and 2 trials, lesinurad has been tested on over 700 patients as a single agent and in combination with allopurinol and febuxostat.