Physicians Often Underestimate the Risk of Opioid Abuse, Misuse, and Diversion in Chronic Pain Patients
Despite rampant opioid misuse, abuse, and diversion self-reported by chronic pain patients in a study presented at the American Pain Society 33rd Annual Scientific Meeting, primary care providers tended to downgrade the patients' risk for engaging in those drug-related aberrant behaviors, indicating a gap between physicians' objective risk assessment for opioid abuse and the actual extent of the problem.
Despite rampant opioid misuse, abuse, and diversion self-reported by chronic pain patients in a study presented at the American Pain Society 33rd Annual Scientific Meeting, held April 30, 2014, to May 3, 2014, in Tampa, FL, primary care providers tended to downgrade the patients’ risk for engaging in those drug-related aberrant behaviors, indicating a gap between physicians’ objective risk assessment for opioid abuse and the actual extent of the problem.
Using data from the ACCESS and ConVERT studies conducted in chronic pain patients receiving Avinza (extended-release morphine sulfate) or Embeda (extended-release morphine sulfate/naltrexone hydrochloride), respectively, Beatrice Setnik, PhD; and Carl L. Roland, PharmD, MS; and Glenn C. Pixton, MS, of Pfizer, Inc., alongside Kenneth W. Sommerville, MD, of the Duke University Medical Center in Durham, NC, “compare(d) investigator assessment of patient risk for behaviors related to prescription opioid misuse, abuse, and diversion with patient self-reports with patient self-reports of these activities and with the objective urine drug testing.”
Although both of the studies utilized patient medical history, urine drug test results, and physician ratings of the patients’ risk levels for aberrant behaviors related to opioids, each implemented different risk assessment questionnaires.
For instance, the investigators in the ACCESS study utilized several tools to determine their patients’ risk for opioid misuse and abuse, including “treatment agreement, card for obtaining/tracking prescriptions, (the) Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R), (and) pill count,” whereas those participating in the ConVERT study completed the Investigator Risk Assessment Questionnaire (IRAQ) at the first visit in order to evaluate the perceived risk of opioid misuse, abuse, and diversion in a given patient and document the sources used to make the risk assessment, the poster authors observed.
On the self-assessment side, patients in the ACCESS study also completed the 24-item SOAPP-R, while those in the ConVERT study filled out the 17-item Current Opioid Misuse Measure (COMM) and the Self-Reported Misuse, Abuse, and Diversion (SR-MAD) questionnaire.
According to Setnik and her co-authors, the investigators in the ACCESS study designated 46.9% of the patients as low risk for aberrant behaviors, 51.8% as moderate, and a mere 1.3% as high risk. Similarly, in the ConVERT study, the investigators labeled the vast majority of the patients as low risk for abuse (89.3%), misuse (84.2%), and diversion (94.3%) — assessments they based “mostly on medical history (84.9%), patient interview (81.7%), and history of treating/knowing the patient (67.8%) … (while) only 21.5% of investigators used questionnaires to assess risk.” Less than 2% of patients were considered high risk for each of those behaviors by the investigators in the ConVERT study.
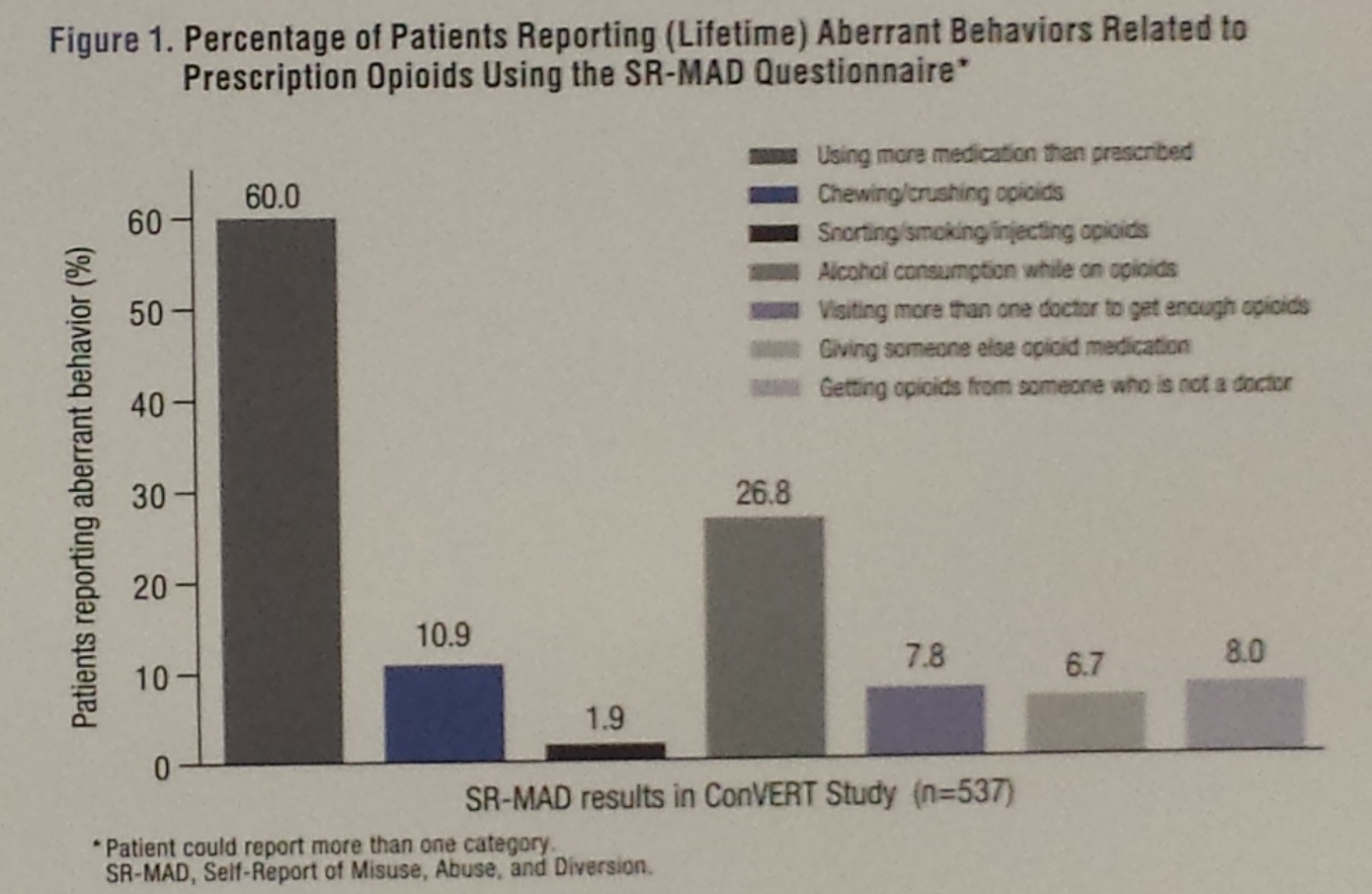
Yet, 24.8% of the patients who completed the SOAPP-R indicated high risk of opioid abuse, misuse, and diversion; 40.6% of COMM responders were categorized as engaging in the drug-related aberrant behaviors; and an astounding 60% of those who filled out the SR-MAD questionnaire admitted using more opioids than prescribed (Figure 1).
In response to those findings, the poster authors suggested that “physicians may require additional education and/or training on the appropriate interpretation and identification of signals resulting from risk assessment tools.”
However, Roland pointed out during the poster presentation that “the way this was set up was under a certificate of confidentially, so the patients were told their physicians would not see the results.” Thus, he said “it would be difficult for physicians to implement these methods in clinical settings.”