Wearable Diabetes Pain Management Device Cleared by FDA
A wearable device for managing diabetic peripheral neuropathy has been cleared by the US Food and Drug Administration.
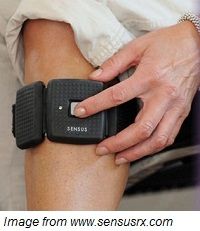
NeuroMetrix announced its wearable, over-the-counter device for chronic pain treatment has been cleared by the US Food and Drug Administration.
The Sensus Pain Management System can be worn by patients during the day while active or at night while asleep. The new device has many purported advantages, according to the manufacturer, including technology that does not allow it to interfere with daily life. The device, which is similar in style to an ankle weight, can be worn under clothing.
The system stimulates the nerves that carry non-painful sensations to the brain, which trigger the release of chemicals that can decrease pain. The stimulations can be increased or decreased as needed, based on the individual’s pain levels. The sessions can last up to one hour and are activated by pushing a button.
The device is powered by a rechargeable lithium-ion battery and creates stimuli with a disposable, snap-in electrode that is changed every 2 weeks.
“Patient response to Sensus, our prescription wearable device for treatment of chronic pain, has been very positive since it was launched in early 2013,” Shai N. Gozani, MD, PhD, President and Chief Executive Officer of NeuroMetrix, said in a press release. “We believe that there is a substantial consumer market for an over-the-counter version of this technology. The ability to offer both prescription and over-the-counter products will give us maximal market exposure and allow us to reach more people with chronic pain.”
Gozani also said between 5 and 6 million people suffer from diabetic peripheral neuropathy and for those who also experience pain the disease can be unbearable. However, he noted that the device can keep users from become sedentary, an effect that may occur due to the previously unmanageable pain. Remaining active can decrease the likelihood of further nerve damage.
“Peripheral neuropathy is the most common complication of diabetes,” he said. “One of the long-term problems is that it severely damages a person’s nerves, which causes many problems including the inability to feel the feet, severe pain or amputation.”
The Sensus system, Gonzani explained in a 2012 interview, is non-addictive with easily-regulated doses and it avoids many of the common side effects of drugs. Patients using the device can monitor its usage and be evaluated by their physician.
The company hopes that the device can also be used to treat other pain and sleep disorders, like fibromyalgia, shingles, and restless leg syndrome. NeuroMetrix anticipates a commercial launch in 2015.