- Revenue Cycle Management
- COVID-19
- Reimbursement
- Diabetes Awareness Month
- Risk Management
- Patient Retention
- Staffing
- Medical Economics® 100th Anniversary
- Coding and documentation
- Business of Endocrinology
- Telehealth
- Physicians Financial News
- Cybersecurity
- Cardiovascular Clinical Consult
- Locum Tenens, brought to you by LocumLife®
- Weight Management
- Business of Women's Health
- Practice Efficiency
- Finance and Wealth
- EHRs
- Remote Patient Monitoring
- Sponsored Webinars
- Medical Technology
- Billing and collections
- Acute Pain Management
- Exclusive Content
- Value-based Care
- Business of Pediatrics
- Concierge Medicine 2.0 by Castle Connolly Private Health Partners
- Practice Growth
- Concierge Medicine
- Business of Cardiology
- Implementing the Topcon Ocular Telehealth Platform
- Malpractice
- Influenza
- Sexual Health
- Chronic Conditions
- Technology
- Legal and Policy
- Money
- Opinion
- Vaccines
- Practice Management
- Patient Relations
- Careers
Eli Lilly is Serious Competition in Diabetes Market
Eli Lilly & Co. is taking a shot at Sanofi with a new drug that matches Sanofi's Lantus at controlling blood sugar, but also helps patients lose weight.

Eli Lilly & Co. is taking a shot at Sanofi with a new drug that could be better than Sanofi’s Lantus. While the two drugs were matched at controlling blood sugar, Lilly’s also helped patients with Type 2 diabetes lose weight.
Developed with Boehringer Ingelheim, LY2605541, is just one of at least four regulatory submissions within the next two years. On Monday, Lilly announced the results of the trial that showed weight lose for type 2 diabetes patients.
Over the course of 12 weeks, patients who took Lilly’s experimental insulin lost an average of 1.28 pounds compared to a weight gain of 0.68 pounds with Lantus. While the weight loss is modest, is could be a commercial advantage for Lilly.
Lilly’s insulin also worked better after eight weeks in patients with type 1 diabetes.
The company’s stock ended the day up 0.36% on Tuesday. Right now it is at the higher end of its 52-week range: $33.75 to $42.03.
Click to enlarge
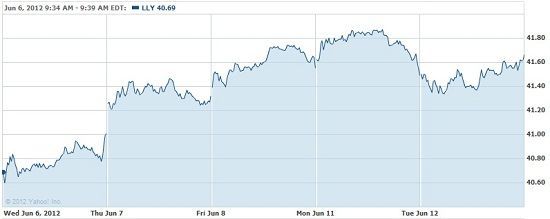
Another drug in the pipeline for Lilly in the diabetes market right now is empagliflozin in Phase III testing. A trial for the drug will complete this year with possible submission in the U.S. and Europe in 2013. Either alone or as an add on to metformin, empagliflozin reduced A1C, fasting plasma glucose and body weight in adults with type 2 diabetes.
Although Lilly has not had a presence in basal analogs, the company is currently working on two in late-stage development. Both are developed with Boehringer and if the trials prove successful, the company expects to submit the products to regulatory authorities in 2013.
The information contained in this article should not be construed as investment advice or as a solicitation to buy or sell any stock.
Read more:
