- Revenue Cycle Management
- COVID-19
- Reimbursement
- Diabetes Awareness Month
- Risk Management
- Patient Retention
- Staffing
- Medical Economics® 100th Anniversary
- Coding and documentation
- Business of Endocrinology
- Telehealth
- Physicians Financial News
- Cybersecurity
- Cardiovascular Clinical Consult
- Locum Tenens, brought to you by LocumLife®
- Weight Management
- Business of Women's Health
- Practice Efficiency
- Finance and Wealth
- EHRs
- Remote Patient Monitoring
- Sponsored Webinars
- Medical Technology
- Billing and collections
- Acute Pain Management
- Exclusive Content
- Value-based Care
- Business of Pediatrics
- Concierge Medicine 2.0 by Castle Connolly Private Health Partners
- Practice Growth
- Concierge Medicine
- Business of Cardiology
- Implementing the Topcon Ocular Telehealth Platform
- Malpractice
- Influenza
- Sexual Health
- Chronic Conditions
- Technology
- Legal and Policy
- Money
- Opinion
- Vaccines
- Practice Management
- Patient Relations
- Careers
First New Weight-Loss Drug Approved in 13 Years
For the first time in 13 years, the U.S Food and Drug Administration (FDA) approved a new prescription weight-loss drug, lorcaserin, sending shares of Arena Pharmaceuticals soaring.

For the first time in 13 years, the U.S Food and Drug Administration (FDA) approved a new prescription weight-loss drug, lorcaserin, sending shares of Arena Pharmaceuticals soaring. The drug will be sold under the name Belviq.
The company’s stock initially shot up 40% to $12.90 on news of the approval and then continued to waver between $11.5 and $12 for the remainder of the day. This sudden increase in stock follows a run up over the past three months in anticipation of today’s decision.
Click to enlarge
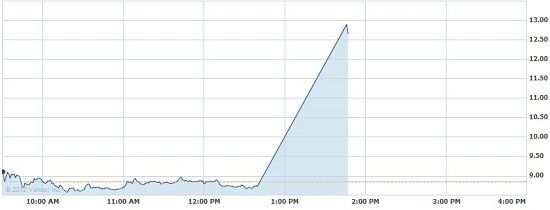
The drug is approved to be used along with diets and increased physicial activity for chronic weight management in obese adults or overweight adults with at least on weight-related condition.
"The FDA approval of Belviq is an important development for patients who struggle with obesity or are overweight with comorbidities and need help with chronic weight management beyond diet and exercise," Jack Lief, Arena's president and chief executive officer, said in a statement.
In trials, patients using Belviq in combination with diet and exercise lost 5% of more of their weight after one year and they managed the weight loss for up to two years.
Common side effects for patients with diabetes were headaches, dizziness, fatigue, nausea, dry mouth and constipation. Patients with diabetes showed hypoglycemia, headaches, back pain, coughing and fatigue.
The FDA approved the drug, which affects the part of the brain that control appetite and metabolism.
“Obesity threatens the overall well being of patients and is a major public health concern,” Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research, said in a statement. “The approval of this drug, used responsibly in combination with a healthy diet and lifestyle, provides a treatment option for Americans who are obese or are overweight and have at least one weight-related comorbid condition.”
Tokyo-based Eisai Co. has been licensed to sell the drug in the U.S. Its stock was up 9.34% on the OTC Markets.
The information contained in this article should not be construed as investment advice or as a solicitation to buy or sell any stock.
Read more:
