Long-term Botox Treatment Sustains Prevention of Chronic Migraine Headaches
Although the benefit of Botox (onabotulinumtoxinA) for preventing headaches in adults with chronic migraine has been extensively established in clinical trials, limited data exists on the injections' effectiveness for the indication beyond 5 treatment cycles.

Although the benefit of Botox (onabotulinumtoxinA) for preventing headaches in adults with chronic migraine has been extensively established in clinical trials, limited data exists on the injections’ effectiveness for the indication beyond 5 treatment cycles.
In an effort to evaluate the durability of long-term onabotulinumtoxinA therapy for chronic migraine, Andrew Michael Blumenfeld, MD, Director of the Headache Center of Southern California in Encinitas, CA, and his colleagues followed 33 adult subjects aged 18-65 years who had suffered at least 15 headaches in a 30-day period and received a minimum of 7 consecutive Botox injection cycles, with an average interval of 12 weeks between injections. The results of the CLARITY pilot study were detailed in a research poster presented at the American Pain Society 33rd Annual Scientific Meeting, held April 30, 2014, to May 3, 2014, in Tampa, FL.
With an objective to determine the sustained effectiveness of onabotulinumtoxinA through 7-9 treatment cycles, Blumenfeld and his co-authors compared the amount of headaches over a 1-month period at time of the 7th Botox injection to baseline and calculated the mean change in pain scores, headache days, and migraine-related disability. In contrast, the Phase 3 Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) study — which served as the basis for the drug’s October 2010 approval from the US Food and Drug Administration (FDA) for the preventive treatment of headaches in adults with chronic migraine — only examined those outcomes for up to 5 injections, or 54 weeks of Botox treatment.
While retrospectively reviewing the medical records of the chronic migraine patients, the investigators found all 33 received the minimum 7 onabotulinumtoxinA injections, though 17 received 8 treatment cycles and another 10 received 9 cycles.
At baseline, the patients suffered an average of 19 migraine headaches in a span of 30 days. In comparison, those who received 7-9 cycles of Botox treatment experienced an average of 6 headaches over the same time period (Table 1). According to the poster authors, that means “clinically meaningful decreases in the number of headache days from baseline were observed at all treatment cycles, (and the) proportion of patients achieving >50% reduction in headache days at cycles 7 and 9 were 85% and 90%, respectively.”
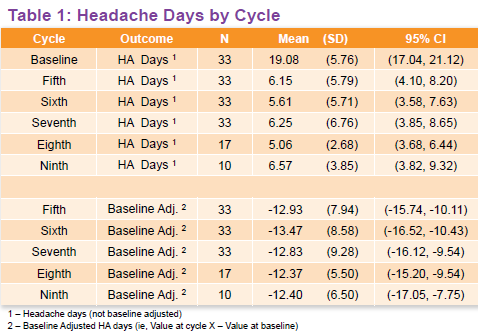
Turning to pain and migraine-related disability scores, Blumenfeld and his colleagues calculated changes in Migraine Disability Assessment (MIDAS), Headache Impact Test-6 (HIT-6), and Patient Health Questionnaire-9 (PHQ-9) results throughout the study period. Similar to the outcome for headache days, the poster authors found a significant decrease from baseline across the MIDAS, HIT-6, PHQ-9 scores, as well as pain measures on a 10-point scale.
Breaking down the data, the researchers said “the mean MIDAS score decreased 31.41 points from baseline to cycle 7, and the mean HIT-6 score decreased 7.50 points from baseline to cycle 7;” additionally, “the mean PHQ-9 score decreased 3.80 points from baseline to cycle 7, and the mean pain score decreased 2.82 points from baseline to cycle 7.” They also pointed out that no serious adverse events were reported throughout the Botox treatment cycles.
“Our results suggest durability of benefit for onabotulinumtoxinA based on the reduction in headache days after 7 treatments, and in this study, through 9 treatments,” Blumenfeld and his co-authors wrote. “OnabotulinumtoxinA demonstrated long-term improvements in migraine-related disability as evaluated by the MIDAS and HIT-6 instruments (and) also demonstrated long-term improvements in pain scores, consistent with other outcomes.”
Although the researchers concluded their study “suggest(s) there was continued effectiveness and durability of benefit of onabotulinumtoxinA after 1 year of treatment,” they noted their findings “warrant investigation in a larger study to better understand the durability of onabotulinumtoxinA in clinical practice for chronic migraine.”
The poster authors disclosed that their research was sponsored by Allergen, Inc., to which Blumenfeld currently acts as a scientific consultant.