Secukinumab Sets New Benchmark in Psoriasis Treatment with 5-Year Phase 3 Data
The drug is the first and only fully-human antagonist showing sustained skin clearance rates at 5 years.
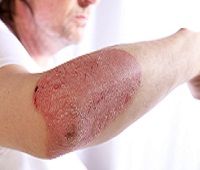
Novartis has announced first of its kind, landmark phase 3 data showing that secukinumab (Cosentyx) set a new benchmark in psoriasis treatment by showing long lasting skin clearance in patients with moderate-to-severe plaque psoriasis at 5 years.
Data from the phase 3 A2304E1 study was presented at the 26th European Academy of Dermatology and Venerology (EADV) Congress in Geneva, Switzerland.
“The 5-year data reinforce Cosentyx as an important treatment option for those people living with psoriasis who aspire for skin clearance that can last,” said Vas Narasimhan, global head, drug development and chief medical officer, Novartis. “Cosentyx is the first and only IL-17A inhibitor approved for psoriasis, psoriatic arthritis and ankylosing spondylitis and has been prescribed to more than 100,000 patients since launch.”
By specifically targeting interleukin-17A (IL-17A), secukinumab addresses key cytokine involved in the development of psoriasis. Inhibiting IL-17A is important as up to 30% of patients with psoriasis have plaque psoriasis and psoriatic arthritis (PsA).
The study is consistent with previous secukinumab studies showing the effectiveness the drug has providing psoriatic arthritics with significant, sustained defense against structural joint damage.
Study A2304E1 is a multicenter, double-blind and open label, 5-year extension to the pivotal phase 3 SCULPTURE study. The objective was to assess the long-term safety, efficacy and tolerability of secukinumab in patients with moderate-to-severe plaque psoriasis.
Effectiveness measures included the proportion of patients achieving a Psoriasis Area and Severity Index (PASI) of 75, 90 and 100, and clear skin. Over the extended treatment period from year 1 (week 52) to the end of year 5 (week 260), response rates remained consistent. PASI 75 and PASI 90 response rates were achieved by 89% and 69% of patients at year 1 and were maintained at year 5. Additionally, 44% of patients achieved completely clear skin, PASI 100, at year 1, with a maintained rate at year 5 (41%).
In SCULPTURE, PASI 75 responders at week 12 were randomized to double-blind maintenance treatment of secukinumab 300 mg or 150 mg, given either at a 4-week, fixed-interval regimen or in a retreatment-as-needed regimen. Those who completed 54 weeks of the SCULPTURE study were eligible to continue the same dose and regimen in the extension study, N=642.
Launching in 2015, Novartis submitted a Biologics License Application (BLA) for a first-in-class human monoclonal antibody (mAb) secukinumab to the US Food and Drug Administration in 2013. More than 100,000 patients worldwide are prescribed secukinumab and 2017 marks 10 years since the first patient and first visit in a clinical trial with secukinumab.
Secukinumab is the first and only fully human IL-17A inhibitor approved to treat psoriasis, PsA and ankylosing spondylitis (AS). It delivers long-lasting skin clearance with proven sustainability — safety out to 5 years — and once-monthly dosing in a patient-friendly auto injector. It’s also approved to treat the more difficult to treat types of plaque psoriasis like palmoplantar psoriasis, scalp psoriasis and nail psoriasis.
In the United States, secukinumab is approved as a the treatment for moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy (light therapy) and in more than 70 countries for active treatment of AS and PsA.
A press release was made available.
Related Coverage
Pfizer Seeks to Expand Tofacitinib Approval to Include Psoriatic Arthritis
Psoriatic Arthritis: Recognizing the Key Features
Valeant Announces US Launch of Brodalumab Injection for Plaque Psoriasis