Upper Leg Stent Approved by FDA for Peripheral Artery Disease
The FDA recently approved the Supera peripheral stent system to treat blocked blood vessels in the upper leg caused by peripheral artery disease.
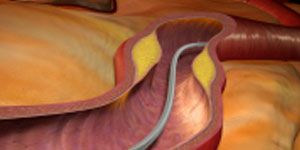
The US Food and Drug Administration (FDA) has approved Abbott’s Supera peripheral stent system to treat blocked upper leg blood vessels caused by peripheral artery disease (PAD), the company announced Friday in a press release.
The stent is an important advancement for PAD patients, the company said, because it mimics rather than restricts the artery’s natural movement, designed to lessen pain. The stent has been specially approved to treat blockages in the superficial femoral arteries (SFA) and the proximal popliteal artery (PPA).
The FDA looked at data from the SUPERB trial as part of the approval process. In the study, Supera was proven to be highly effective in opening up blocked blood vessels with lasting results. After the first year of treatment, there were no stent fractures, and at 2 years the fracture rate was 0.5%.
“Doctors are increasingly identifying peripheral artery disease as a major cause of leg pain, which can limit people’s ability to live a healthy lifestyle,” Kenneth Rosenfield, MD, section head of Vascular Medicine and Intervention at Massachusetts General Hospital and the study’s principal investigator, said in a statement.“Treatment with the Supera stent, as shown by the results of the SUPERB study, is very effective in easing leg pain, enabling the majority of patients to resume their activities.”