Adapalene, Benzoyl Peroxide Gel Shown to Help Prevent Further Scarring from Acne Vulgaris
Given that this new research was an exploratory study with a smaller sample size, the study’s results may not be generalized and future studies should use more patients and institutions.
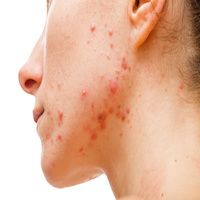
Adapalene 0.1%/benzoyl peroxide 2.5% (ADP/BPO) gel and benzoyl peroxide 2.5% gel maintenance therapy is efficacious in preventing scar increases among individuals with acne vulgaris, according to new findings.1
This new research was designed to explore the ways in which maintenance therapy can impact atrophic scarring, given the scarcity of data on ADP/BPO gel and BPO gel’s role in treating patients with acne vulgaris in clinical practice settings.
The research was led by Nobukazu Hayashi, from the Department of Dermatology at Toranomon Hospital in Tokyo, Japan. Hayashi and colleagues noted that a prior study found that those treated with ADP/BPO gel for a total of 6 months ended up with diminished atrophic scarring risk as well as scar severity improvements.2
Given that the ways in which maintenance therapy may impact atrophic scarring had not been completely explored, this new study “aimed to evaluate the efficacy of maintenance therapy with ADP/BPO gel and BPO gel and its effects on atrophic scarring using 3D image analysis,” Hayashi and colleagues wrote.
Background and Findings
The investigators focused on a cohort of adult study participants who were aged 20 years and older, and this group had been diagnosed with acne vulgaris. The participants had exhibited a diminished number of inflammatory lesions to 10 or fewer at the time of the acute-inflammatory-phase treatment in a 3-month span prior to giving their informed consent.
In addition, the participants were required to have between 10 and fewer than 100 atrophic scars, with each scar being 0.5 mm minimum in its diameter. Before participant enrollment, the investigators had a qualified investigator carefully elaborate on the research objectives and the procedures to each participant, collecting individual consent for their participation.
The investigators’ research was done as a multicenter, open, randomized intergroup comparison research initiative and held at 13 different medical institutions around Japan, taking place from August 2020 to September 2021. Both the enrollment and the allocation of subjects were done through an electronic data capture (EDC) system.
Allocation was dynamically performed using a minimization method, maintaining a ratio of 1:1:1 between ADP/BPO gel, BPO gel, and a control group without maintenance treatment drugs. The allocation adjustment factor was based on the acute-inflammatory-phase drugs, which included treatments combining ADP and BPO, treatments with only BPO, treatments with only ADP, and other alternative treatments.
The team, over the course of a 24-week time frame, provided the prescribed treatment to those assigned to the ADP/BPO gel and BPO gel arms of the study. The subjects were required to apply the study drug to the affected areas once daily before sleeping following the investigator's instructions.
In all arms of the study, a moisturizing agent was also utilized as necessary per the research team's guidance. The team strictly prohibited participants’ use of specific therapies, drugs, and cosmetics during the research timeframe to ensure integrity.
Overall, the investigators first divided the 126 participants into 3 distinct groups: ADP/BPO with 40 individuals, BPO alone with 44, and a control group without maintenance treatment drugs with 42. After a 24-week period, 111 of them were able to finish the study.
The team’s primary outcome, which was defined as keeping the number of inflammatory lesions at ≤10, was found to be 89.2% among those in the ADP/BPO arm, 87.5% among the BPO peroxide arm, and 47.4% among those in the control arm. They also found that both treatment arms had substantially higher rates of success compared to those in the control arm (P = 0.0006 for both).
As a secondary outcome, the 2 treatment groups ended up showing major improvements in the rate of change in their atrophic scarring from baseline at 24 weeks (P = 0.0004 and P < 0.0001, respectively). Although 3-dimensional image analysis parameters stayed stable in the treatment groups, the control arm showed substantial deterioration (P = 0.0276 for affected area, P = 0.0445 for volume, and P = 0.0182 for maximum depth).
Additionally, the investigators noted that adverse drug reactions were seen among 7.5% of the participants in the ADP/BPO arm, but none took place within the BPO group.
“The above findings suggest that maintenance therapy using ADP/BPO gel and BPO gel may be effective in preventing the relapse of inflammatory lesions in Japanese patients with acne vulgaris,” they wrote. “Furthermore, the objective evaluation of acne scars has revealed that the therapy reduced atrophic scars without aggravating them.”
References
- Tanizaki, H, Hayashi, N, Abe, M. Evaluation of the efficacy of maintenance therapy for acne vulgaris using adapalene 0.1%/benzoyl peroxide 2.5% gel and benzoyl peroxide 2.5% gel for 24 weeks and assessment of atrophic acne scars using three-dimensional image analysis. J Dermatol. 2023; 00: 1–10. https://doi.org/10.1111/1346-8138.16942.
- Dreno B, Tan J, Rivier M, Martel P, Bissonnette R. Adapalene 0.1%/benzoyl peroxide 2.5% gel reduces the risk of atrophic scar formation in moderate inflammatory acne: a split-face randomized controlled trial. J Eur Acad Dermatol Venereol. 2017; 31: 737–742.