Article
A Biosimilar Cure for Sky-High Drug Prices
Author(s):
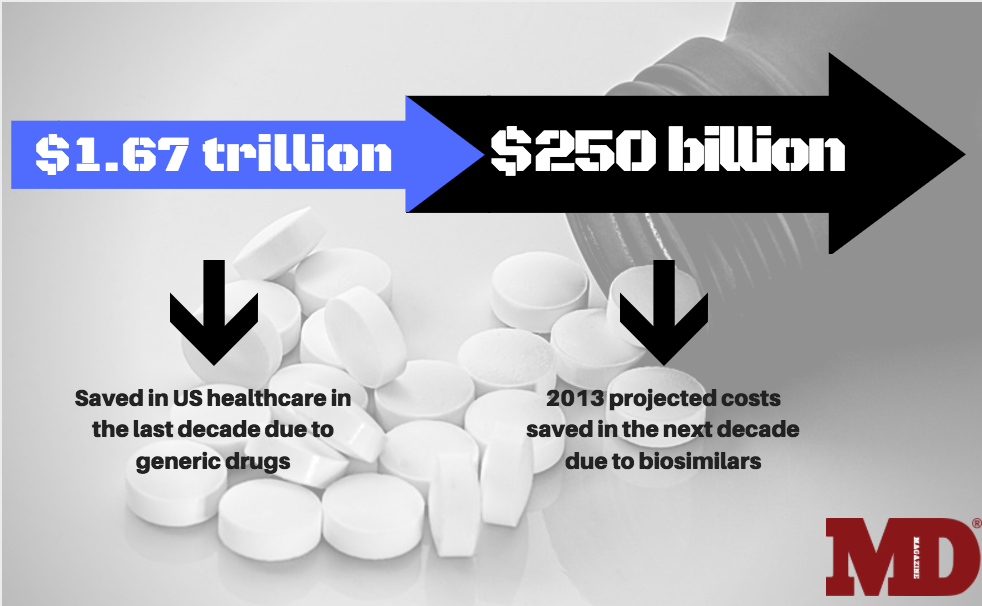
In 2013, it was estimated that because of biosimilars, the US could save $250 billion throughout the next 10 years.
A decade after the introduction of the first biosimilar into the market, Mvasi — the country’s first-ever biosimilar cancer drug — was approved just last week.
Biosimilars are approved based on a showing that the drug is highly similar to an already-approved biological product and has no clinically meaningful difference in terms of safety, purity and potency from the reference product, in addition to meeting other criteria specified by law.
The US Food and Drug Administration (FDA) has spoken on bringing a new emphasis to bringing biosimilars onto the market since Scott Gottlieb, MD, was named commissioner this year.
“Bringing new biosimilars to patients, especially for diseases where the cost of existing treatments can be high, is an important way to help spur competition that can lower healthcare costs and increase access to important therapies,” Gottlieb said earlier this year. “We’ll continue to work hard to ensure that biosimilar medications are brought to the market quickly, through a process that makes certain that these new medicines meet the FDA’s rigorous gold standard for safety and effectiveness."
Over the past few years, biosimilar research skyrocketed courtesy to the passage of the Affordable Care Act (ACA), which included a pathway for biosimilars to become FDA-approved in 2010. The goal was to provide patients access to cheaper versions of costly drugs made from living cells, which until the law, didn’t have a path for replicas.
Today, many of the world's largest drugmakers are researching biosimilars, and the approval of Mvasi is a testament to their success. The first-ever biosimilar cancer treatment’s approval is a victory with huge implications for cancer drug prices.
An increase of biosimilars in the United States could drive down the cost of biologic medications. At the recent European Society of Medical Oncology, experts said the price of biosimilars are expected to be about 30% lower than that of reference products.
In 2013, it was estimated that the US could save $250 billion throughout the next 10 years because of biosimilars. As prescription drug costs continue to rise, biosimilars have great potential to lower costs improving patient access to life-saving medicines.
Generic versions of traditional pills can cost pennies compared to brand-name versions, saving the US health system $1.67 trillion in the last decade, reports the Association for Accessible Medicines (AAM).
The AAM and its biosimilar council released an analysis finding that if the Centers for Medicare and Medicaid Services (CMS) were to revise its current reimbursement policy for biosimiliar medicines, the federal government could potentially save $11.4 billion on medicines throughout the next 10 years.
“Encouraging more competition through options like biosimilars, is one way we can help contain or maybe even reduce costs, while spurring innovation for new therapies,” said Sheila Frame, vice president and head of biopharmaceuticals, Sandoz, North America.
In June, the FDA took steps to increase competition in the market, facilitating entry of lower-cost alternatives. The agency published a list of off-patent, off-exclusivity branded drugs without approved generics, and implemented for the first time, a new policy to expedite the review of generic drug applications where competition is limited.
While biosimilars are expected to be more cost effective than the brands, they require more testing and manufacturing resources.
Many biological medicines are coming off patent, and much cheaper biosimilars are becoming available, resulting in the price of the original drug falling dramatically.
“Competition from these life-saving and life-enhancing medicines is already helping the National Health Service (NHS) treat more patients and this will only increase as further biosimilar medicines become available,” Warwick Smith, director general of British Biosimiliars Association said. “As more biosimilar medicines become available to treat complex diseases such as cancer, expect uptake to accelerate further as clinicians and prescribers are more used to working with these medicines, which are proven to have no clinically meaningful differences to the originator product in terms of safety, efficacy and quality.”
Of the 7 FDA-approved biosimilar drugs since 2015, only 3 are available for sale, while the other 4 remain unavailable — mired in court proceedings that can block the biosimilars for years.
It’s feared that drugmakers could use the patent system to delay biosimilar versions of biologics for extended lengths of time using lengthy, strategic patent litigation to artificially extend the effective length of a brand’s exclusivity, resulting in higher costs.
The FDA is attempting to better familiarize doctors with biosimilars and considering changes to draft guidelines on how makers of biosimilar drugs can prove to the FDA that doctors can switch patients from 1 drug to another. In the following months, the FDA will be launching a public service campaign to educate physicians on the biosimilars process.