Article
Biotech Firm Gets $688K to Build In Vitro Blood-Brain Barrier
Author(s):
A Seattle company is hoping its technology will help drug companies more efficiently test out the safety and efficacy of drugs that must cross the blood-brain barrier.
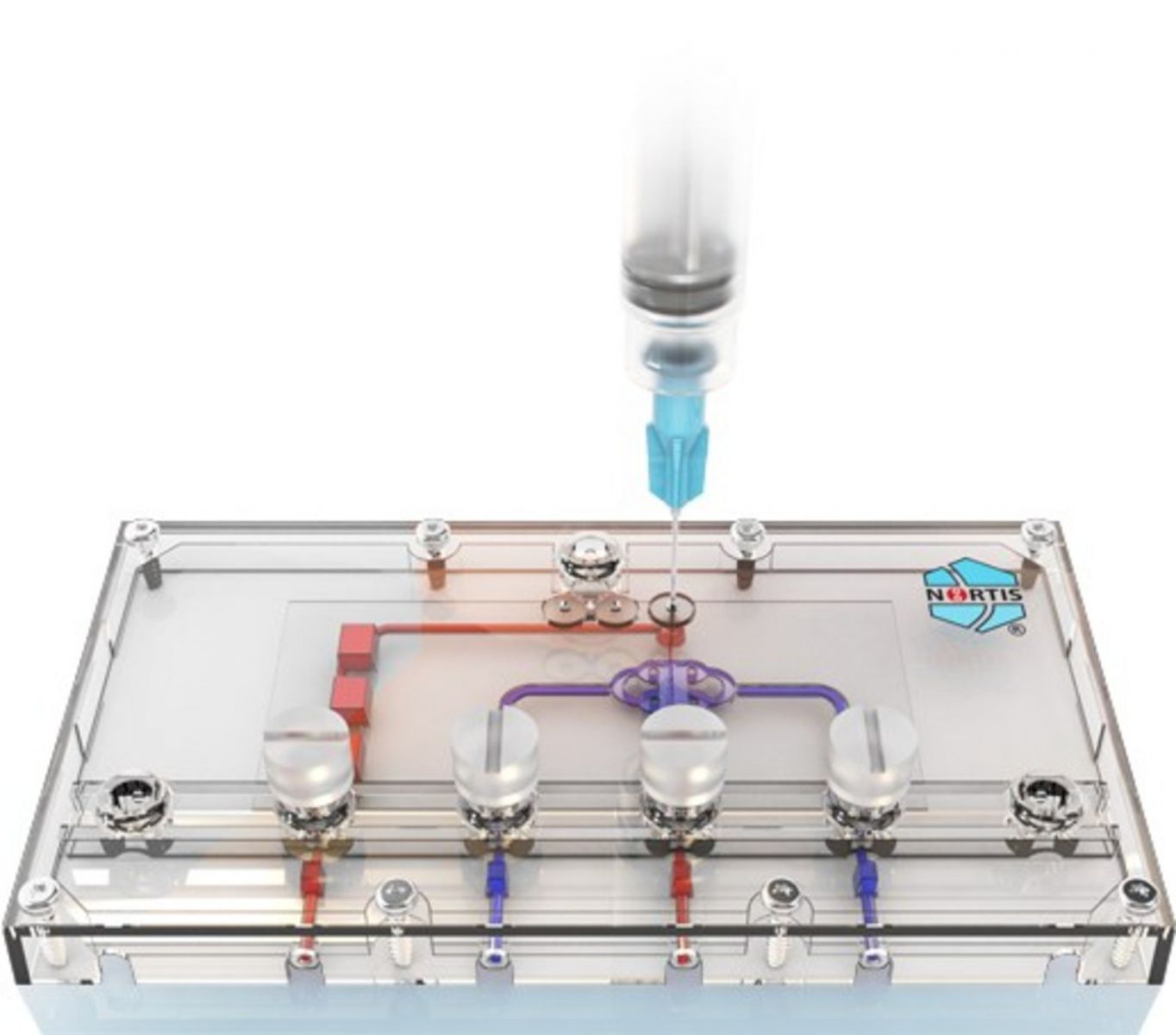
A Seattle-based biotechnology company has received a new round of federal funding that will allow it to develop an in vitro testing system that mimics the human blood-brain barrier.
Nortis Inc. was awarded a $688,000 grant from the National Institutes of Health. The money will fund the third year of a Small Business Innovation Research fast-track grant from the National Institute of Neurological Disorders and Stroke.
Nortis has developed a proprietary 3D organ modeling system called ParVivo, which regulates the biological structure of human organs to allow accurate in vitro testing. The creation of a blood-brain barrier in vitro model could have a major impact on how drug companies test and develop treatments for neurological disorders like multiple sclerosis (MS).
Kevin Banks, PhD, Nortis’ vice president of sales and marketing, told MD Magazine that the technology dovetails nicely with efforts by drug developers to reduce animal testing and lower the rate of clinical trial failures. Banks noted that about 4 of every 10 Phase III studies are failures, a ratio that translates to expensive losses for drug companies.
“This is primarily due to efficacy and safety failures of the drugs, which have been primarily tested to that point using animal models and not human models,” Banks said. “The vision of using human 3D tissue models is to reduce clinical trial failure rates by using more relevant human models early in the drug development process.”
Drug delivery to the brain has long been a challenge for pharmaceutical companies. The blood-brain barrier, which is composed of endothelial tissue, protects the brain from foreign substances and helps maintain a constant environment, but the barrier also can stop drug molecules from getting into the brain.
Thus, companies developing drugs that require entry into the brain to trigger the desired pharmacological response have a major interest in efficiently and reliably figuring out how their therapies hold up against the barrier.
Banks said the ParVivo system allows researchers to develop “far more complex tissue models compared to more simple 3D tissue printing techniques.”
The system also has perfusion capability, the ability to run a single assay on the system while doing up to 72 tests at the same time, and the opportunity to do the drug discovery and toxicity testing in-house instead of having to outsource it. However, Banks said Nortis has also launched a contract research service for clients that desire that service.
Banks said the company recently returned from the 3D Tissue Models 2017 Show in Boston, where they found significant interest from drug developers. Overall, he said, there’s been a mix of interest levels from drug companies.
“Like with any new technology, it depends on the company,” Banks said. “We have early adopter organizations actively using the ParVivo system in drug mechanism discovery efforts, in addition to others evaluating the use of the platform for more accurate and human relevant toxicology studies.”
Banks said he feels the company has made it through the “innovator adoption” phase and is now fully into the “early adopter” phase for ParVivo. The new grant money will help the company build technology to help new clients, including those developing drugs to treat MS.
Related Coverage
Iron Intake Could Contribute to Pediatric Multiple Sclerosis
Mavenclad Wins EU Approval After Reversal; FDA Application Coming Soon





