Clinical Review: Brimonidine Gel for Treatment of Facial Erythema in Rosacea Patients
Rosacea affects approximately 16 million people in the United States, and patients often present with a variety of symptoms and need an individualized treatment plan.
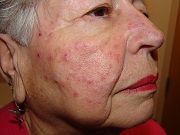
Rosacea affects approximately 16 million people in the United States, and patients often present with a variety of symptoms and need an individualized treatment plan. Rosacea can affect the facial skin and, in about 1 in 5 cases, also the eyes, causing redness, irritation, and a burning sensation. There is no cure for rosacea.
Most treatments are intended to control the signs and symptoms that define the disease, and while those treatments are generally safe and effective, there are few medications that are FDA-approved to treat rosacea. Treatments for all four subtypes of the disease generally target inflammatory lesions, leaving a treatment gap in options to treat the persistent facial erythema that can occur in all subtypes.
A recent review article in Dermatology and Therapy looked at possible combination therapies with brimonidine tartrate 0.33% gel, approved in 2013 by the FDA for the topical treatment of persistent facial erythema associated with rosacea. Brimonidine gel is a highly selective α2-adrenergic receptor agonist (with an established safety profile over 20 years of treating open-angle glaucoma) that leads to significant reduction of persistent facial erythema in the majority of patients when applied once daily.
In large-scale clinical trials, brimonidine has been shown to be generally safe and effective, with adverse effects that are mild-to-moderate in degree and which often resolve spontaneously with continued use.
According to the study authors, “Significant improvement in erythema is experienced in the majority of patients with once-daily topical application with few experiencing mild to moderate cutaneous adverse effects that are often not persistent with continued use. This provides clinicians with a safe and effective treatment modality for this previously difficult to treat manifestation of rosacea, and it has been shown to be safely used in combination with other therapeutics targeting the inflammatory lesions of the disease.”
Importantly, the review took an in-depth look at the case reports of “exaggerated recurrence” or “rebound” associated with the use of topical brimonidine tartrate gel. Several reports have shown cases in which persistent erythema was favorably reduced within one to 6 hours after application, only to be followed by an “exaggerated recurrence” of erythemapast baseline at 12 hours after application, which lasted an additional 12 hours. Evidence as part of the review shows that,
“There is usually rapid resolution of these AEs with only rare reports of paradoxical erythema or exaggerated recurrence of erythema with the aforementioned associated symptoms lasting weeks, and in a majority of cases the AE improved or resolved after stopping brimonidine… the incidence of all AEs decreased from quarter one through quarter four over the first 12 months of daily use.”
Brimonidine gel is intended to be applied as a pea-sized amount once daily to each of five regions of the face: the central forehead, chin, nose, and each cheek, with even application as a thin layer avoiding the eyes and lips.
The FDA notes that brimonidine tartrate topical gel should be used with caution in patients with depression, cerebral or coronary artery insufficiency, and patients with severe or unstable cardiovascular disease, as α2-adrenergic agonists can lower blood pressure. The gel should also be kept out of the reach of children.
The review also includes anecdotal evidence from the study authors about lessening of adverse effects through reinforcing good skin hygiene with all patients suffering from rosacea, including using only gentle face cleansers, using only the fingers to cleanse the face, and avoiding the use of toners, astringents, washcloths, or other abrasive products.
Further studies are needed to address continued long-term use of this medication, the authors note, including the open question of “whether its use may permanently alter neurovascular mechanisms behind persistent erythema in rosacea and possibly alter the course of this clinical manifestation over time.”