Early SLE often Misdiagnosed for Mimics
Patients with systemic lupus erythematosus often have a range of clinical symptoms that are nonspecific and overlap with other medical conditions, such as Sjögren’s syndrome, rheumatoid arthritis and Raynaud's syndrome.
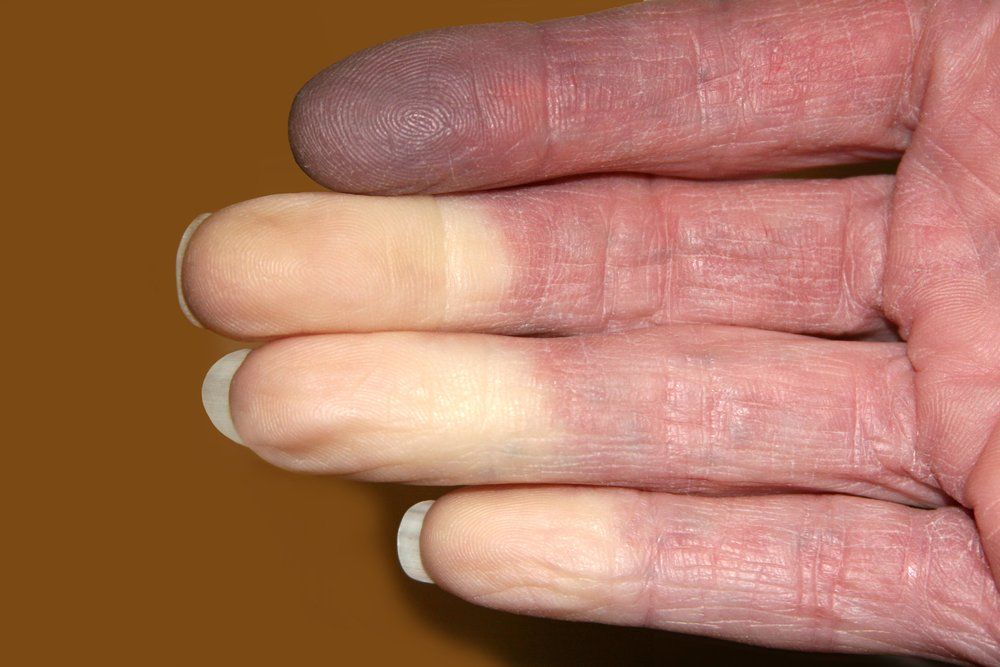
Patients with systemic lupus erythematosus often have a range of symptoms, such as Raynaud's syndrome as shown here. (©TwinschoiceShutterstock.com)
A new study suggests that testing positive for antinuclear antibodies (ANA) may be an important first step for differentiating early systemic lupus erythematosus (SLE) from mimicking conditions. Symptoms like fatigue, dysphagia and Raynaud’s phenomenon may steer physicians towards a different diagnosis than SLE, especially when ANA is negative.
Results could be used to improve the accuracy of current classification criteria for the diagnosis of SLE. The brief report was published in the January issue ofArthritis & Rheumatology.
“The early diagnosis of SLE may have a great impact on disease outcomes as it could allow early treatment and damage prevention. Therefore, it is important to identify clinical and serological features that may raise the suspicion of SLE and could lead to an earlier diagnosis,” said first author Marta Mosca, M.D., Ph.D., of the University of Pisa, Italy.
Yet, the initial diagnosis of SLE can be tricky. Individuals with SLE often have a range of clinical symptoms that are nonspecific and overlap with other medical conditions like Sjögren’s syndrome, rheumatoid arthritis, and other autoimmune conditions. Onset is often insidious, and many patients have subclinical symptoms for years before diagnosis. Moreover, SLE has a relapsing-remitting course, so that not all individuals exhibit all of the symptoms all of the time.
Finally, diagnostic criteria developed by the American College of Rheumatology (ACR) and the Systemic Lupus International Collaborating Clinics (SLICC) leave room for improvement, and may be less accurate for patients with early disease.
The study took place in seven academic medical centers in Asia, Europe, North Americana and South America, and included patients referred to lupus specialty clinics for further evaluation. Of these, 389 turned out to have early SLE and 227 had conditions that mimic SLE.
Next: The study
Researchers compared clinical symptoms and serology test results for patients who received a diagnosis of early SLE, vs those with mimicking conditions. SLE-mimickers included undifferentiated connective tissue disease, Sjögren’s syndrome, systemic sclerosis, primary Raynaud’s phenomenon, fibromyalgia, ANA-positive thyroiditis, rheumatoid arthritis, mixed connective tissue disease, hematologic diseases, infections, autoimmune hepatitis, psoriatic arthritis, and miscellaneous conditions like rosacea, osteoarthritis, and erythema nodosum.
The analysis also assessed the diagnostic accuracy of 1997 ACR and 2012 SLICC SLE classification criteria, compared to diagnosis by a lupus specialist (considered gold standard).
Results showed that a significantly higher percentage of patients with early SLE had noninfectious fever compared to those with SLE-mimicking conditions (34.5% versus 13.7%, respectively; P < 0.001).
Those with early SLE were also more likely to have alopecia (30.6% versus 11.9%, respectively; P < 0.001), weight loss (13.1% versus 4.4%; P < 0.001), and ascites (3.1% versus 0%; P = 0.005).
On the other hand, some symptoms were significantly more common among patients with SLE mimicking conditions. These included: Raynaud’s phenomenon (48.5% vs 22.1% ; P < 0.001), sicca symptoms (34.4% vs 4.4%; P < 0.001), dysphagia (6.2% vs 0.3%; P < 0.001), and fatigue (37.0% vs 28.3%; P = 0.024).
Among lab tests, those with early SLE were significantly more likely to test positive for ANA (99.5% vs 95.1%; p<0.001), and to have antibodies to anti–double-stranded DNA (anti-dsDNA: 71.7% vs 6.9%; p<0.001), and Smith (anti-Sm: 30.2% vs 2.6%; p<0.001).
Other lab tests more commonly abnormal with early SLE included: anticardiolipin IgM (IgM aCL: 13.2% vs 2.0%; <0.001); anti–β2-glycoprotein I antibodies (Anti-β2 GPI: 17% vs 4.4%; p=0.001); positive Coombs’ test (12.3% vs 5.7%; p=0.008); autoimmune hemolytic anemia (4.6% vs 0.4%; p=0.003), hypocomplementemia (73.4% vs 48.4%; p<0.001), and leukopenia (16.2% vs 9.8%; p=0.02).
Patients with early SLE vs SLE mimicking conditions showed no significant differences for antibodies to Ro (anti-Ro: 33.2% vs 25.6%; p=0.06) and La (anti-La: 15.1% vs 9.9%; p=0.09), suggesting that these tests are not helpful in differentiating the two groups.
Further analysis suggested that SLICC 2012 was more accurate than ACR 1997 (82.1% vs 75.5%, respectively). About 34% of patients diagnosed with early SLE did not meet ACR criteria, while 16.5% of those with early SLE did not meet SLICC criteria.
Nevertheless, Dr. Mosca concluded: “The comparison of clinical manifestations presented by patients with early SLE vs mimicking conditions, has shown that standard items of existing classification criteria for SLE are more prevalent in SLE than in mimicking conditions, as might be expected.”
REFERENCES
Mosca M, Costenbader KH, Johnson SR, et al. Brief Report: How Do Patients With Newly Diagnosed Systemic Lupus Erythematosus Present? A Multicenter Cohort of Early Systemic Lupus Erythematosus to Inform the Development of New Classification Criteria. Arthritis Rheumatol. 2019 Jan;71(1):91-98. doi: 10.1002/art.40674.
Inês L, Silva C, Galindo M, et al. Classification of Systemic Lupus Erythematosus: Systemic Lupus International Collaborating Clinics Versus American College of Rheumatology Criteria. A Comparative Study of 2,055 Patients From a Real-Life, International Systemic Lupus Erythematosus Cohort. Arthritis Care Res (Hoboken). 2015 Aug;67(8):1180-5. doi: 10.1002/acr.22539.