Article
FDA Approves Non-Invasive Stimulation Device for Cluster Headache
Author(s):
Previous studies link vagus nerve stimulation to pain relief for various conditions.
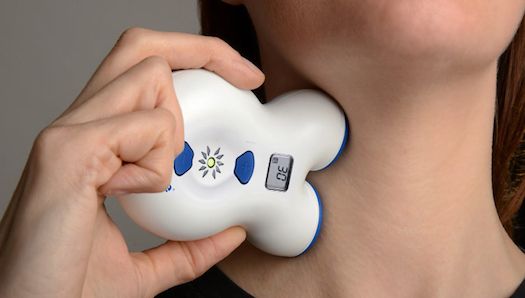
electroCore, a Basking Ridge, New Jersey-based maker of medical devices, today announced the United States Food and Drug Administration’s acceptance of their gammacore device for the treatment of cluster headache. The unit, pictured above in intended use, transmits mild electrical stimulation to the vagus nerve through the skin in order to alleviate pain.
Cluster headache is a relatively rare, typically intense headache felt behind the eyes. The condition has a quick onset, and electroCore hopes their product can just as quickly reverse the pain associated with it. In prospective, double-blind, placebo-controlled, randomized studies of the device, gammaCore performed well. The ACT 1 trial (ACute Treatment of Cluster Headache) found that 34.2% of patients using the device had their headache pain reduced to a “mild” status or none at all within 15 minutes of initiating use of the device, compared to 10.6% of patients treated with placebo (p=0.008).
In ACT 2, 27 patients with a combined 182 cluster headache attacks were studied. The chasm between the gammaCore group and the placebo group was even wider, with 47.5% of attacks deemed pain-free after 15 minutes of treatment compared with 6.2% in placebo (p=0.003).
Francis R. Amato, CEO of electroCore, was quoted with great confidence in the company’s official press release, saying that his company is “leading the way for the future of medicine through the development of patient-administered, non-invasive vagus nerve stimulation therapy.”
The vagus nerve is responsible for heart rate, sweating, gastrointestinal peristalsis, and several muscle movements of the mouth including speech. Its primary function is to supply motor parasympathetic fibers to all the organs from the neck down to the second segment of the transverse colon, except the adrenal glands. Other studies have found that stimulating it may have a positive effect on pain from various conditions. In August of 2016, a Proceedings of the Natural Academy of Sciences study endorsed the use of non-invasive vagus nerve stimulation for the alleviation of rheumatoid arthritis symptoms. Another study presented at last year’s American College of Cardiology annual meeting indicated that such electrical stimulation may benefit heart failure patients.
Proponents of the gammaCore device champion the fact that it’s non-invasive and can be easily used by patients in their own homes as needed. “It does not have the side effects or dose limitations of commonly prescribed treatments or the need for invasive implantation procedures, which can be inconvenient, costly and high-risk," the official press release quotes Stephen Silberstein, MD, who is Director of the Headache Center at Jefferson University in Philadelphia.
electroCore expects to have the device commercially available early in the third quarter of this year.
Related Coverage:
Vagus Nerve Stimulation Shows Some Benefit in Heart Failure
Vagal Nerve Stimulation Device Shows Promise in Treating Chronic Migraine





