Identifying and managing osteoporosis: An update
Osteoporosis is associated with significant morbidity and mortality. The fracture risk assessment tool (FRAX) calculates the contribution of significant clinical risk factors and bone mineral density to estimate the 10-year risk of major osteoporotic fracture and hip fracture.
Osteoporosis ranks with hypertension, hypercholesterolemia, and diabetes mellitus (DM) as one of the most common chronic diseases in the United States.1 More than 10 million Americans have osteoporosis and 33.6 million have low bone mass at the hip, according to estimates from the Third National Health and Nutrition Examination Survey.2
Significant morbidity and mortality are associated with osteoporosis. Fifty percent of white women and 20% of white men will have at least 1 fracture resulting from osteoporosis.3 The probability that a 50-year-old person will have a hip fracture during his or her lifetime is 14% for a white woman, 5% to 6% for a white man, 6% for an African American woman, and 3% for an African American man.4 The rate of mortality in the year after a hip fracture is 24%.5
Osteoporosis also constitutes a major public health issue. More than 2 million fractures resulting from osteoporosis accounted for an estimated $19 billion in health care costs in 2005 alone.5 The annual cost is predicted to rise to $25.3 billion by 2025.
The fracture risk assessment tool (FRAX), recently developed by the World Health Organization (WHO), calculates the contribution of significant clinical risk factors and bone mineral density (BMD) to estimate the 10-year risk of major osteoporotic (vertebral, hip, forearm, or humerus) fracture and hip fracture.6 Using the FRAX algorithm; applying current National Osteoporosis Foundation (NOF) recommendations for testing, preventing, and managing osteoporosis; and correcting vitamin D deficiency provides clinicians with the potential to identify and manage osteoporosis and significantly improve the health and quality of life of patients with this disease.
In this article, we offer a definition of osteoporosis, describe how the diagnosis is made, discuss the role that vitamin D plays, and address when to manage and what to do with osteopenia. We also describe recommendations for treatment and monitoring of patients with osteoporosis.
Defining osteoporosis
Osteoporosis may be defined as inadequate bone strength that places a person at risk for fragility fractures, typically fractures of the wrist, hip, and spine resulting from minimal trauma (eg, a fall from standing height). Bone strength is made up of 2 components, bone density and bone quality.
Bone density may be measured with dual-energy x-ray absorptiometry (DXA); it is the basis of the WHO classification of osteopenia and osteoporosis according to T-scores. The areal BMD in g/cm2 measured by DXA is compared with the mean BMD of a young normal adult of the same sex to derive a T-score expressed as 1 standard deviation above or below the mean (Table 1).
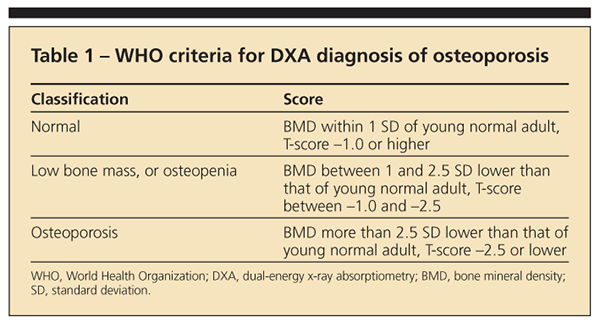
Bone quality involves several parameters, including collagen quality, mineralization, and microarchitecture (eg, the thickness and intact nature of the struts of the trabeculae or cortex). Bone quality cannot be measured directly except by bone biopsy, but it may be inferred indirectly by the occurrence of a fragility fracture.
Making the diagnosis
The diagnosis may be made based on the occurrence of a fragility fracture of the hip or spine or on WHO criteria applied to the hip, spine, or distal forearm. Although other techniques for assessing BMD are available (eg, ultrasonography and quantitative CT), they cannot be used for diagnosis because T-scores obtained with these devices were derived from different populations and do not correlate with T-scores on central DXA; also, WHO criteria cannot be used.7
Routine DXA testing measures the hip and spine; the forearm may be added if spine osteoarthritis or hip replacements limit measurements of these sites or if secondary loss of cortical bone (eg, hyperparathyroidism, vitamin D deficiency) is suspected. The NOF recommends DXA testing for all women 65 years and older. Other persons in the NOF recommendations for testing include the following8:
•Men 70 years or older, regardless of clinical risk factors.
•Postmenopausal women younger than 65 years and men aged 50
to 69 years who have clinical risk factors.
•Women in menopausal transition if there is a specific factor associated with increased fracture risk, such as low body weight.
•Adults older than 50 years who have a fracture.
•Adults who have a condition or are taking a medication associated with low bone mass or bone loss.
Prevention
All low bone mass being caused by loss of bone is a common misconception. Low bone mass often occurs as a result of low peak bone mass. If there are no secondary causes of bone loss, a young person with low peak bone mass is not at significant risk for fracture. However, he is at risk for osteoporosis sooner when bone loss and microarchitectural deterioration begin around menopause (or after age 50 years in men).
Therefore, prevention should be aimed first at increasing peak bone mass. Good health measures, including adequate intake of calcium and vitamin D, are important for reaching peak bone mass, particularly during the adolescent years-85% to 90% of adult bone mass is acquired by age 18 years in girls and 20 years in boys.5
For adults, the NOF recommends regular weight-bearing exercise to maintain BMD and muscle-strengthening exercises to reduce the risk of falls and fractures. Intake of calcium, 1200 mg/d, and vitamin D, 800 to1000 IU/d, is recommended for all adults. Clinicians should advise against tobacco use and excessive alcohol intake.8
The role of vitamin D
1,25-Dihydroxyvitamin D (1,25[OH]2D) is not a vitamin but rather a hormone that is involved in multiple physiological processes. There are vitamin D receptors on most cells in the body; deficiency not only affects bone health and causes proximal muscle weakness but also has been linked with multiple diseases, including colon, prostate, and breast cancer; multiple sclerosis; hypertension; rheumatoid arthritis; systemic lupus erythematosus; type I DM; tuberculosis; and schizophrenia.9
Vitamin D deficiency is important in osteoporosis because it can cause both low bone mass and increased fall risk. Deficiency causes decreased calcium absorption, leading to a secondary elevation of parathyroid hormone (PTH) levels and increased bone resorption. It is associated with increased body sway and falls, which also increases fracture risk.9
The results of clinical trials suggest that vitamin D supplementation may decrease fractures. In a meta-analysis of 12 randomized clinical trials, intake of 700 to 800 IU/d of vitamin D3 was associated with a 26% decreased relative risk of hip fracture; however, there was no significant benefit of taking 400 IU/d.9 Given this information, adequate vitamin D intake should be considered an essential component of any prevention or treatment regimen for patients with osteoporosis.
Despite the importance of vitamin D, deficiency is extremely common in persons of all ages and in all ethnic groups. An expert panel stated that the “minimum desirable 25-hydroxyvitamin D concentration clusters between 28 and 32 ng/mL” are considered optimal for bone health,10 yet levels lower than 20 ng/mL have been seen in multiple studies, including in 48% to 52% of healthy children and adolescents in Maine and Boston; 42% of African Americans aged 15 to 49 years; and 73% of pregnant and lactating women, even though they were taking a prenatal vitamin with 400 IU of vitamin D daily.9 Even in clinical trials of patients taking medication for osteoporosis, more than 50% had a vitamin D level lower than 30 ng/mL, including those living in sunny climates.9
Vitamin D recommendations
The Institute of Medicine recommends daily intake of vitamin D at 200 IU for children and adults younger than 50 years, 400 IU for adults aged 50 to 70 years, and 600 IU for adults 71 years and older.11 However, it is clear now that these levels are not sufficient. In 2008, the American Academy of Pediatrics increased its recommendation for vitamin D intake to 400 IU/d for infants through adolescents. The NOF recommends intake of 800 to 1000 IU/d for all adults older than 50 years.8 Higher doses may be required for patients at high risk for vitamin D deficiency, including older and homebound persons, those in ethnic groups with dark skin, obese persons (fat tissue sequesters vitamin D), and persons with malabsorption (eg, those who have celiac sprue, Crohn disease, or bariatric surgery).
To determine vitamin D status, 25-hydroxyvitamin D (25[OH]D) levels should be measured. Although 1,25(OH)2D is the active hormone, it fluctuates significantly in response to other hormones and minerals and measuring it in clinical practice is not useful, except in chronic renal disease. The NOF recommends maintaining serum 25(OH)D levels at least as high as 30 ng/mL.
Mild vitamin D deficiency may be managed with increased daily doses of vitamin D3 (cholecalciferol, available over the counter). If the level is lower than 20 ng/mL, once-weekly doses of 50,000 IU of vitamin D2 (ergocalciferol, available by prescription) for 8 to 12 weeks usually results in a normal 25(OH)D level, which then can be maintained with 1000 IU/d of vitamin D3.
The safe upper limit for vitamin D intake was set at 2000 IU/d in 1997,11 but recent evidence suggests that higher levels are safe. In a review of 37 studies, toxic vitamin D levels were seen only when the dose exceeded 10,000 IU/d and published cases of symptomatic vitamin D toxicity all involved intake of at least 40,000 IU/d.12
When to treat and what to do with osteopenia
The American College of Physicians recommends that clinicians offer pharmacological treatment to men and women who have known osteoporosis and to those who have experienced fragility fractures.13 A DXA diagnosis of osteoporosis is an indication to consider pharmacological treatment, but making the diagnosis of osteoporosis on the basis of DXA alone misses many patients who go on to fracture. In fact, most fractures occur in patients whose T-scores are higher than –2.5.14,15
Pasco and associates15 monitored 616 postmenopausal women for 5 years; 73.1% of fractures occurred in women who did not have osteoporosis (56.5% had osteopenia and 16.6% had normal BMD) by measurement at the femoral neck or total hip. This large group of patients with osteopenia clearly represents a population the treatment of whom probably will prevent the largest number of fractures. However, there is significant diversity of fracture risk within this gray area of BMD, making it difficult for clinicians to choose appropriate patients for treatment.
In 1999, the first NOF guidelines recommended treatment for patients with T-scores lower than –1.5 with 1 or more significant risk factors. However, this approach is difficult to use in clinical practice for several reasons.
One problem is that there is no universally accepted list of risk factors. Risk factors may be endocrine related, including hormone deficiency in women (amenorrhea resulting from excessive exercise or eating disorders and aromatase inhibitors in patients with breast cancer) or in men (primary testosterone deficiency and androgen deprivation therapy for prostate cancer), type I DM, hyperthyroidism, and hyperparathyroidism.
Other major risk factors include GI, renal, and oncological diseases (celiac disease, inflammatory bowel disease, renal calcium wasting, and multiple myeloma), as well as organ transplantation. Lifestyle factors include cigarette smoking; intake of alcohol and caffeine; vitamin D deficiency; and use of prescribed medications (corticosteroids and anticonvulsants).
Most of these risk factors affect BMD, but there are other risk factors for fracture (eg, fall risk) that are difficult to quantify. This large list of potential risk factors can be cumbersome and difficult to use in clinical practice.
Another problem is that not all risk factors increase fracture risk to the same extent. For example, drinking more than 3 cups of coffee a day does not increase risk the same as having a maternal history of hip fracture.
FRAX algorithm advantages
In an attempt to quantify risk factors, the WHO evaluated multiple multinational, prospective epidemiological trials to develop the FRAX algorithm.6 Risk factors include age, body mass index, sex, personal history of fracture as an adult, parental hip fracture, previous use of corticosteroids, intake of more than 3 alcoholic beverages per day, current cigarette smoking, rheumatoid arthritis, and other secondary causes. Although this list does not include all known risk factors, these factors were shown in multiple prospective epidemiological studies to most accurately predict future fracture.
The FRAX analysis has many advantages (Table 2), particularly the ability to assess fracture risk in

patients with osteopenia, who constitute the majority of patients seen by clinicians and have the most fractures.13,14 Knowing the actual fracture risk in these patients helps physicians make clinical decisions. A 10-year fracture risk is easier for patients and their physicians to understand than a T-score and can be useful in risk-benefit analyses. Use of the FRAX algorithm may increase awareness and prevention efforts for men and persons in ethnic groups. The Framingham data on DM, coronary artery disease, and hypertension have guided therapy for hypercholesterolemia; the FRAX algorithm can similarly guide decision making for patients with osteopenia or osteoporosis.
FRAX limitations
As with any tool, the FRAX analysis has limitations. The algorithm was developed from epidemiological data on postmenopausal women and men older than 50 years and cannot be applied to premenopausal women or younger men. Fracture risk in premenopausal patients or men younger than 50 years with low bone mass is not known and may be low because they have not experienced the microarchitectural deterioration that occurs with menopause and the aging process.
Fracture risk cannot be calculated in patients who are receiving treatment for osteoporosis, particularly bisphosphonates, because pharmacological therapy reduces fracture risk even when there is no change in BMD. Current FRAX calculations use BMD or T-score of the femoral neck only because the clinical databases of BMD and fracture risk on which FRAX is based do not include other sites. However, the NOF has stated that the T-score of the total hip can be substituted.8 Fracture data are not adequate to use BMD of the spine.
In addition, risk factors (except for age and body mass index) are listed as dichotomous variables, but many factors have a gradient of risk. Although in the FRAX algorithm patients with 3 vertebral fractures have the same calculated risk as patients with a wrist fracture, they clearly have different risks of future fracture. Similarly, a patient who is receiving 60 mg/d of prednisone does not have the same risk as a patient who received 5 mg/d of prednisone for 3 months 2 years ago. FRAX should be used as a means to guide treatment decisions, but it is not a substitute for clinical judgment.
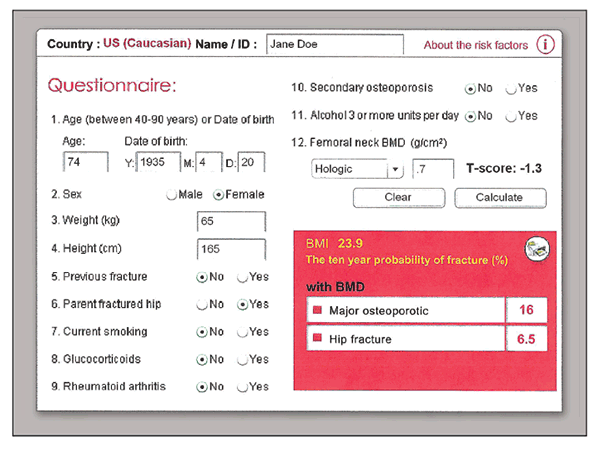
Figure 1 – This fracture risk assessment tool calculation was done for a 74-year-old woman with osteopenia and a parental history of hip fracture. She would not be a candidate for treatment according to the original National Osteoporosis Foundation criteria, but she meets the new guidelines.
Figure 1 illustrates a sample risk assessment using the most recent 3.0 version of FRAX in a 74-year-old woman with a T-score in the osteopenic range, –1.3. According to the original NOF criteria, she would not be a candidate for treatment. However, with a parental history of hip fracture and her age, she clearly meets the new NOF guidelines for treatment (hip fracture risk greater than 3%).
Results not equally applicable
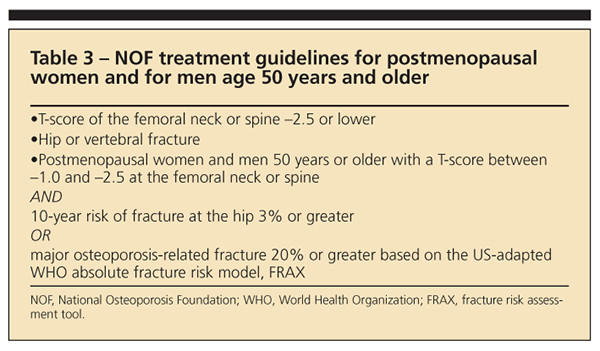
Although the FRAX algorithm may simplify treatment decisions in many patients, the results may not be equally applicable for all patients. Therapy always should be considered for patients with T-scores lower than –2.5 or fragility fractures regardless of calculated 10-year fracture risk (Table 3).
Other clinical issues often need to be considered. For example, in a 75-year-old patient with multiple
myeloma and a limited life expectancy, the fracture risk will be overestimated with FRAX. A 55-year-old woman just entering menopause with a T-score of –2.3 has a low 10-year fracture risk but may have a high lifetime risk; pharmacological therapy to prevent postmenopausal bone loss might be considered. The use of FRAX does not preclude clinical judgment for treatment of individual patients.
Treating patients with osteoporosis
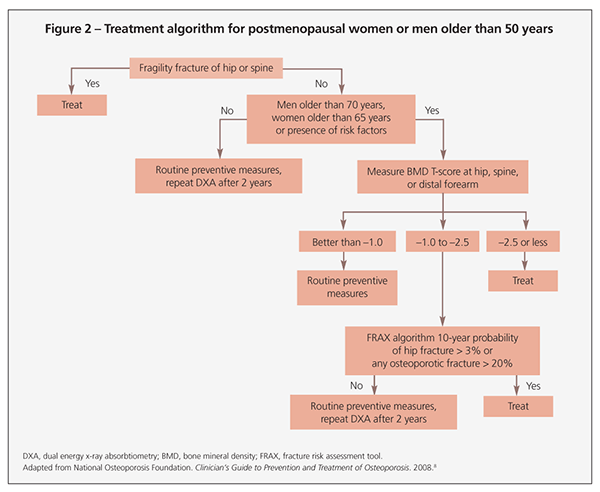
Treatment recommendations always should include adequate intake of calcium and vitamin D, exercise, and general health measures. Additional therapy should be considered if the NOF indications in Table 3 are present. Figure 2 offers a treatment algorithm for pharmacological therapy based on current NOF recommendations.
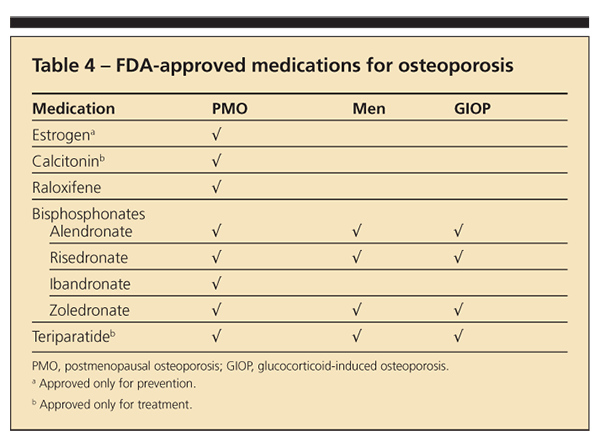
Current FDA-approved choices for therapy are shown in Table 4. Estrogen is approved only for prevention, but in the Women’s Health Initiative it was shown to improve BMD and decrease
hip and spine fractures. Raloxifene is approved for the prevention of breast cancer and may be a good choice for younger postmenopausal patients.
Bisphosphonates, available in oral and intravenous forms, are the main choice of therapy for patients with osteoporosis. Teriparatide, the only anabolic agent, is reserved for patients at significant risk for fracture. Many other agents are in clinical trials.
Monitoring patients
The NOF recommends repeated BMD scans using Medicare guidelines: every 2 years or 1 year
after patients change therapy to identify those who are not responding to treatment. Nonresponse is defined as loss of bone; fracture alone (particularly a single fracture) does not constitute a diagnosis of failure, because none of the current medications prevents all fractures.
If bone loss is noted, the first step is to review the DXA scan carefully to ensure that there has been a true loss of BMD that exceeds the least significant change of the
machine.7 If there has been a loss, patient compliance should be
assessed.
As in many chronic diseases, compliance is a significant problem in osteoporosis. A systematic review of 14 databases showed a 1-year persistence rate with bisphosphonates ranging from 17.9% to 78% and a compliance rate defined as a medication possession ratio from 0.59 to 0.81.16 Although persistence was better for weekly than for daily bisphosphonates (35.7% to 69.7% vs 26.1% to 55.7%), it remained inadequate in most patients. Measuring bone turnover markers may be helpful in assessing compliance: resorption markers, such as N-telopeptide or C-telopeptide, should be suppressed in patients who are receiving adequate antiresorptive therapy.
If the patient has been compliant with therapy, a workup for secondary causes of bone loss is appropriate because unexpected secondary causes of bone loss are not uncommon. In a study of 173 women with osteoporosis in which no secondary causes were noted on initial evaluation, additional laboratory evaluation revealed secondary causes in 32% of participants.17 Vitamin D deficiency was the most common finding in this study; other occult causes to consider include celiac disease, multiple myeloma, hypercalciuria, and hyperparathyroidism.
References:
References1. Melton LJ 3rd. How many women have osteoporosis now? J Bone Miner Res.1995;10:175-177.
2. National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002.
3. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Office of the Surgeon General; 2004.
4. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. JAMA. 2001;285:785-795.
5. National Osteoporosis Foundation. Fast Facts on Osteoporosis. http://www.nof.org/professionals/Fast_Facts_Osteoporosis. Accessed December 17, 2009.
6. Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385-397.
7. International Society for Clinical Densitometry. Official Positions. http://www.iscd.org/Visitors/positions/OfficialPositionsText.cfm. Accessed December 17, 2009.
8. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington DC: National Osteoporosis Foundation; 2008. http://www.nof.org. Accessed December 17, 2009.
9. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
10. Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713-716.
11. Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997.
12. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842-856.
13. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404-415.
14. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study [published correction appears in Bone. 2006;38:603]. Bone. 2004;34:195-202.
15. Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporosis Int. 2006;17:1404-1409.
16. Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023-1031.
17. Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431-4437.