Joint Aspiration and Injection: A Look at the Basics
Joint aspiration may be used for diagnosis or for relieving pressure, and joint injection may be used for treatment. Physicians can easily become proficient in aspiration and injection techniques. Indications for aspiration include both acute and chronic arthritis; there are few absolute contraindications. Intra-articular injections of medication usually are an adjunct to other treatment modalities.
ABSTRACT: Joint aspiration may be used for diagnosis or for relieving pressure, and joint injection may be used for treatment. Physicians can easily become proficient in aspiration and injection techniques. Indications for aspiration include both acute and chronic arthritis; there are few absolute contraindications. Intra-articular injections of medication usually are an adjunct to other treatment modalities. Indications for corticosteroid injections include acute crystal-induced arthritis; complications are rare. Less soluble agents remain in a joint space longer than more soluble agents and should have a longer duration of action. Physicians should perform injections wearing gloves and using aseptic technique. No one technique has proved to be optimal. After an aspiration or injection, the patient should be given detailed instructions. (J Musculoskel Med. 2011;28:216-222)
Two primary indications call for inserting a needle into a joint: (1) aspiration of fluid for diagnostic purposes or for relieving pressure within a swollen joint and (2) injection of medications. Joint aspiration and intra-articular injection are useful and somewhat safe procedures that physicians who treat patients with musculoskeletal conditions can perform readily at the bedside. Clinicians usually can learn and become proficient in aspiration and injection techniques in a limited amount of time. In appropriate cases, joint injections are a valuable adjunct to medical therapies, rehabilitation, and surgery.
In this article, we address the indications and contraindications for joint aspiration and intra-articular injection. We also discuss the potential complications, available therapies, technical aspects of injection, and the possible role of ultrasonography-guided procedures.
Indications and contraindications
for joint aspiration
Diagnostic indications include acute arthritis (sepsis, crystal arthritis [monosodium urate; calcium pyrophosphate; basic calcium phosphates, such as hydroxyapatite; oxalate; cholesterol], and hemorrhagic [trauma]) and chronic arthritis (inflammatory [crystal arthritis, rheumatoid arthritis, spondyloarthritis] and noninflammatory [osteoarthritis, osteonecrosis]). Treatment indications include reduction of intra-articular pressure, injection of medication (local anesthetic, corticosteroids, hyaluronic acid, and other agents [radioisotopes, such as yttrium; infliximab]), repeated aspiration for sepsis, and saline.

It could be argued that joint aspiration should be performed in any patient who presents with a painful joint or joints and evidence of effusion of unknown causes. Synovial fluid analysis is one of the most sensitive and inexpensive investigations for differentiating various pathologies, including infection, immune-mediated inflammation, crystal-induced inflammation, trauma, and neoplasm. The gross appearance and viscosity of the synovial fluid and, most important, the total white blood cell (WBC) count with differential can reliably determine the inflammatory or noninflammatory nature of the underlying condition (Table 1).
In addition, crystalline arthritis (gout or pseudogout) may be readily confirmed by examining the fluid under a compensated, polarizing microscope, and a Gram stain and culture of the fluid may be diagnostic for septic arthritis. Removal of fluid temporarily alleviates acute symptoms and improves joint mobility by reducing intra-articular pressure. However, it probably will not provide prolonged relief unless targeted therapy is used for the underlying condition.1
There are few if any absolute contraindications for simple aspiration of a joint. A joint should not be aspirated if the overlying skin has a large purulent ulcer; however, cellulitis overlying a swollen joint is not a contraindication if it is the only portal for intra-articular access. The risk of introducing infection by aspirating the joint through cellulitic skin is far less than the risk of septic arthritis going unmanaged.
In addition, a major coagulopathy (eg, hemophilia) is not an absolute contraindication for aspiration of a swollen joint, because if left unmanaged, a hemarthrosis can rapidly destroy a joint. It can lead to intra-articular damage caused by toxic effects of blood products, resulting in synovial hypertrophy, fibrosis, and impaired joint movement. Repeated attacks or persistent hemorrhage for more than 6 months leads to chronic disabling arthropathy. These situations require physicians to pay careful attention to aseptic precautions and hemostasis achieved through adequate pressure, respectively.
For patients who are receiving long-term anticoagulation therapy, the international normalized ratio (INR) should be within their ideal therapeutic range before joint aspiration. One study described patients as being at low risk if their INR was lower than 3.7.2 If possible, avoid using a needle larger than 22 gauge in patients who are receiving anticoagulation therapy.
Injection therapy:
General principles
Intra-articular injections of medication usually are an adjunct to other specific treatment modalities for joint diseases. Generally, a joint should be aspirated for the presence of fluid before injecting any medication. Aspiration of pus (purulent effusion) should prompt an immediate evaluation by Gram stain and culture and, therefore, prohibit an injection. In daily clinical practice, a physician may not wait for the final culture results, depending on the clinical scenario and how well he or she knows the patient. However, routinely sending the fluid for culture could avoid potential future complications.
The variety of available intra-articular therapies includes local anesthetics; several types of corticosteroids; hyaluronic acid preparations; and radionucleotides, such as yttrium. Immediate pain relief after local anesthetic injections may confirm a joint to be the source of pain (this “diagnostic test” usually is used when pain originating in a deep-seated joint, such as the hip or sacroiliac joint, is suspected) as well as the proper place for the injection.3
Injection therapy:Corticosteroids
Indications for corticosteroid injections include treatment of acute crystal-induced arthritis as well as a “flare” of chronic inflammatory arthritis, such as rheumatoid arthritis (RA). Treatment of knee osteoarthritis (OA) probably is the most common and the best-studied use of intra-articular corticosteroid injections, although the role of inflammation in OA remains controversial.
Two meta-analyses found a favorable effect on pain in patients with OA after intra-articular corticosteroid injections.4,5 However, the symptomatic improvement was short-term (1 to 4 weeks); by 8 weeks, there was no difference in target pain reduction between the treatment and control groups.5 The types and dosages of the corticosteroid preparations used, removal of excess fluid, injection technique, and adherence to a postinjection rest period all may have accounted for these differences.1
Systemic bacteremia or suspected septic arthritis is an absolute contraindication to intra-articular corticosteroid injections. Corticosteroids should not be injected into an unstable joint or into a prosthetic joint; finger joints have such inherent instability-being supported by only a few tenuous ligaments-that they should not receive repeated injections. In addition, patients should not receive repeated injections if previous injections resulted in a poor response. A poor clinical response to 2 previous injections in the same joint 3 months apart would constitute a relative contraindication for a third injection in that joint.
Injection complications
Proper informed consent-including a discussion about potential risks, complications, alternatives, and outcomes-should be obtained from each patient and then documented in the record before arthrocentesis or joint injection is performed. Complications of intra-articular corticosteroid injections are rare. Potential complications include joint infection, subcutaneous fat atrophy, skin atrophy or depigmentation, asymptomatic pericapsular calcification, facial flushing, and postinjection flare. The risk of causing septic arthritis is extremely low; however, this condition remains the most feared complication.
Gray and Gottlieb6 reported 2 cases of infection in more than 100,000 injections; Hollander7 described 18 cases in more than 250,000 injections. A postinjection flare, thought to be a result of corticosteroid crystal-induced inflammation, may occur in up to 15% of patients8; it may be confused with the occurrence of septic arthritis. Septic arthritis usually appears 3 to 4 days after arthrocentesis; in contrast, a postinjection flare tends to occur within the first 24 hours and dissipates, on average, within 3 days.
Although there is some evidence of systemic corticosteroid absorption after intra-articular injection, it probably will not be of major clinical significance.8 Occasional use of intra-articular corticosteroids does not appear to contribute to osteoporosis because they have little impact on bone resorption.9
The impact of corticosteroids on glucose control might be another concern. However, a small study in which patients with diabetes mellitus received soft tissue injections of methylprednisolone acetate did not detect a significant effect on blood glucose levels.10
Local adverse reactions to injection are minor and reversible. Subcutaneous fat and skin atrophy and depigmentation may occur, especially at superficial sites. Skin atrophy often reverses over subsequent months but may persist. A rare complication of repeated injections, pericapsular calcification, has been seen on x-ray films. The clinical significance of this finding is unknown. Flushing may occur shortly after corticosteroid injection but usually is self-limited.
Use of intra-articular corticosteroid injections has raised concerns about more joint degradation resulting from increased use of a less painful but diseased joint. Although rabbit studies have reported that use of intra-articular corticosteroids resulted in degeneration of mature cartilage cells,11 this finding has not been supported by studies on primate joints.12
In clinical practice, repeated knee injections with corticosteroids do not appear to result in joint destruction or accelerated deterioration.13 In fact, one study demonstrated a chondroprotective effect of intra-articular corticosteroids that reduced osteoarthritic changes.14 The mechanism of this chondroprotective action remains uncertain.
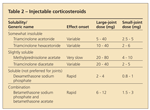
Available products
Corticosteroid preparations differ in their solubility, potency, and crystalline structure (Table 2). In a survey of American College of Rheumatology (ACR) members, methylprednisolone acetate was reported to be the most commonly used injectable corticosteroid, followed by triamcinolone hexacetonide and triamcinolone acetonide.15 Less soluble agents (eg, triamcinolone hexacetonide) remain in a joint space longer than more soluble agents (eg, betamethasone sodium phosphate) and, in theory, should have a longer duration of action or prolonged effect.16
This does not always seem to be the case, however, as shown in a clinical trial of patients with OA. In one study, methylprednisolone acetate had a prolonged clinical effect on pain when compared with triamcinolone hexacetonide, a less soluble compound.17 Less soluble, fluorinated agents generally are used for joint injections only and should be avoided in soft tissue injections because they have higher potential for causing tissue atrophy, tendon rupture, and depigmentation.
Dosage and injection
technique
Physicians should perform all joint and soft tissue injections wearing gloves and using aseptic technique. Although sterile gloves and sterile precautions often are used,18 the gloves need not be sterile if the approach is a “no-touch” technique. In this approach, once the injection site is cleaned with antiseptic solutions, it should not be touched or palpated for anatomical landmarks. The operator enters the needle through the cleaned skin without touching that skin. The dose of the corticosteroid and its potential effect are influenced by various factors, including the size of
the joint, choice of corticosteroid preparation, and severity of inflammation (see Table 2).
Necessary supplies include a patient table, gloves, antiseptic swabs (alcohol, topical iodophor microbicide, chlorhexidine gluconate), sterile needles (19-, 21-, 22-, and 25-guage), sterile syringes (1-, 3-, 6-, 10-, and 20-mL), sterile gauze pads, single-dose sealed vials of corticosteroid, single-dose sealed vials of anesthetic (lidocaine 1%), sterile test tube or container for synovial fluid culture, test tube with sodium heparin for synovial fluid WBC count, slide with cover slip, nail polish (to seal edges of cover slip to view slide at a later time), and hemostat. Surface anatomy and joint landmarks should be identified, and the point of entry can be marked before skin cleansing with a small indentation from a needle cap or thumbnail.
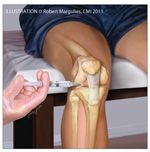
Figure –
The "sitting down" approach to knee injection is easy to do and convenient. The patient sits in a chair with the knee at a right angle. The patella is "pulled up" on the femur and the leg hangs because of gravity; therefore, the knee joint is "opened up." In that position, it can be injected by entering on the medial or lateral side of the patellar tendon. The needle is positioned parallel to the ground.
Aspiration of large joints, such as the knee (Figure) and shoulder, should be done with a 20- or 21-gauge needle; if purulent fluid is present, a larger-gauge needle may be used. After joint aspiration is complete, the same needle may be used for injections (changing the syringe but leaving the needle in place). For planned joint injections without aspiration, a 22- or even a 25-gauge needle is appropriate, depending on the size of the joint.
The choice of syringe size is based on the size of the effusion. Smaller syringes tend to be easier to use for aspiration than larger syringes and often are easier to disengage and reuse.
Most injections may be performed with a needle 1 to 1½ inches long, although a ½-inch needle often is used for the small joints of the hands and feet. Small-joint injection also may be very useful in RA when there is chronic synovitis of a single or maybe 2 small joints. A 3-inch spinal needle may be required for a deeper injection, such as that in the hip joint or trochanteric bursa. Hip aspirations are almost always done with fluoroscopic guidance.
Some physicians use a “2-step” technique: they use a 25-gauge needle to inject the skin or surrounding tissue with local anesthetic and then inject a corticosteroid (alone or mixed with an anesthetic) with a separate needle and syringe into the target joint.16 Others use a “1-step” method of injecting a local anesthetic/corticosteroid mixture into the selected area; although the skin is not anesthetized, the needle enters the target site directly.
Mixing an anesthetic with the corticosteroid in a syringe may allow for better dispersion of the mixture into the joint space. Mixing lidocaine with some corticosteroid preparations may result in a visible precipitant in the syringe that is thought to occur in the presence of paraben preservatives; however, this precipitant is of no clinical significance. Some physicians use 1 needle and syringe to inject the local anesthetic and, while leaving the needle in place, change syringes to introduce the corticosteroid or corticosteroid/lidocaine mixture.
Although there are several techniques, no one has proved to be optimal. The ACR recommends that if a joint is injected with a corticosteroid, aspiration of fluid should precede the injection.19 In a study of patients with RA, aspiration before corticosteroid injection resulted in fewer relapses over a 6-month period.20
After an aspiration or injection, the patient should be given detailed instructions and a list of potential warning signs. Ice packs and anti-inflammatory medications may help prevent postinjection flares. Rest and a reduction in weight bearing for several days, followed by progressive exercises often are recommended.21 Resting helps alleviate symptoms and avoid overuse of the affected joint. Traditionally, limiting injections of large, weight-bearing joints to 4 per year and spacing them at least 4 weeks apart has been recommended, although the scientific reasoning behind this recommendation is uncertain.21
Utility of musculoskeletal
ultrasonography
Ultrasonography has become increasingly popular in musculoskeletal medicine for guidance of aspirations and injections. This modality may be used before the procedure to identify the site of injection before introduction of the needle or in real time to verify placement of the needle with direct visualization. In addition to potentially improving injection accuracy, it has been proposed that ultrasonography confers an advantage by improving the success of aspiration and injection of joints that are otherwise difficult to access by the conventional palpation technique.
One study found the accuracy of blind injections of the shoulder and knee to be 25% and 70%, respectively.22 A study that compared conventional aspirations with ultrasonography-guided aspirations found successful aspiration in 25% vs 100% of the shoulders, 40% vs 95% of the knees, 20% vs 100% of the ankles, and 0% vs 100% of the small joints.23 Overall, the rate of unintended non–intra-articular injections performed by skilled rheumatologists and orthopedic surgeons using a conventional palpation technique has been estimated to be as high as 50% to 60%24; it is hoped that ultrasonography-guided injections will make this number much smaller.
Sibbitt and associates24 conducted a study of 148 patients with direct comparison between conventional palpation-guided and ultrasonography-guided methods; an acceptable clinical response rate of 71.6% was seen in the palpation-guided group. However, the ultrasonography-guided procedures were significantly superior in all outcome measures, including reduction in pain from baseline, procedural pain, and pain at outcome 2 weeks postprocedure, and increase in percentages of responders and decrease in percentages of nonresponders. In addition, ultrasonography increased the detection of effusion by 200% and volume of fluid aspirated by 337%. No difference in safety was demonstrated, and there were noted disadvantages of increased time for ultrasonography-guided procedures as well as increased cost. Extensive cost-benefit analysis still needs to be conducted to determine whether the improvements noted from ultrasonographic guidance can be justified in the long term.
References:
References1. Schumacher HR Jr. Aspiration and injection therapies for joints. Arthritis Rheum. 2003;49:413-420.
2. Thumboo J, O’Duffy JD. A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium. Arthritis Rheum. 1998;41:736-739.
3. Creamer P, Hunt M, Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intraarticular anesthetic. J Rheumatol. 1996;23:1031-1036.
4. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869.
5. Godwin M, Dawes M. Intra-articular steroid injections for painful knees: systematic review with meta-analysis [published correction appears in Can Fam Physician. 2009;55:590]. Can Fam Physician. 2004;50:241-248.
6. Gray RG, Gottlieb NL. Intra-articular corticosteroids: an updated assessment. Clin Orthop Relat Res. 1983;177:235-263.
7. Hollander JL. Intra-articular hydrocortisone in arthritis and allied conditions; a summary of two years’ clinical experience. J Bone Joint Surg. 1953;35A:983-990.
8. Courtney P, Doherty M. Joint aspiration and injection. Best Pract Res Clin Rheumatol. 2005;19:345-369.
9. Emkey RD, Lindsay R, Lyssy J, et al. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:277-282.
10. Slotkoff A, Clauw D, Nashel D. Effect of soft tissue corticosteroid injection on glucose control in diabetics [abstract]. Arthritis Rheum. 1994;37(suppl 9):S347.
11. Papachristou G, Anagnostou S, Katsorhis T. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop Scand Suppl. 1997;275:132-134.
12. Gibson T, Burry HC, Poswillo D, Glass J. Effect of intra-articular corticosteroid injections on primate cartilage. Ann Rheum Dis. 1977;36:74-79.
13. Balch HW, Gibson JM, El-Ghobarey AF, et al. Repeated corticosteroid injections into knee joints. Rheumatol Rehabil. 1977;16:137-140.
14. Pelletier JP, Martel-Pelletier J. Protective effects of corticosteroids on cartilage lesions and osteophyte formation in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum. 1989;32:181-193.
15. Centeno LM, Moore ME. Preferred intraarticular corticosteroids and associated practice: a survey of members of the American College of Rheumatology. Arthritis Care Res. 1994;7:151-155.
16. White R. Supplies and equipment needed for joint injection. In: Pfenninger JL, ed. The Clinics Atlas of Office Procedures: Joint Injection Techniques. Philadelphia: WB Saunders Company; 2002;5:403-412.
17. Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clin Rheumatol. 2004;23:116-120.
18. Charalambous CP, Tryfonidis M, Sadiq S, et al. Septic arthritis following intra-articular steroid injection of the knee-a survey of current practice regarding antiseptic technique used during intra-articular steroid injection of the knee. Clin Rheumatol. 2003;22:386-390.
19. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905-1915.
20. Weitoft T, Uddenfeldt P. Importance of synovial fluid aspiration when injecting intra-articular corticosteroids. Ann Rheum Dis. 2000;59:233-235.
21. Paluska S. Indications, contraindications, and overview for aspirating or injecting a joint or related structure. In: Pfenninger JL, ed. The Clinics Atlas of Office Procedures. Philadelphia: WB Saunders Company; 2002;5:412-422.
22. Jones A, Regan M, Ledingham J, et al. Importance of placement of intra-articular steroid injection. BMJ. 1993;307:1329-1330.
23. Balint PV, Kane D, Hunter J, et al. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: a pilot study. J Rheumatol. 2002;29:2209-2213.
24. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.