Launching the Era of Personalized Medicine in Lung Cancer
Lung cancer, one of the most molecularly complex cancers, is rapidly yielding its secrets. Inside of 10 years, a one-size-fits-all disease and treatment model has been overtaken by a concept of lung cancer as a group of heterogeneous diseases with different molecular origins. Research, drug development, and guidelines are beginning to reflect and amplify a new lung cancer paradigm, in which testing for a “driver” mutation is factored into the selection of therapy, along with the cancer histology.
The history of the epidermal growth factor receptor (EGFR)-targeted therapy erlotinib (Tarceva, OSI Pharmaceuticals)1 was an important factor that helped foster the idea of identifying and directing a therapy to its most likely responders, according to D. Ross Camidge, MD, PhD, from the University of Colorado Comprehensive Cancer Center, who spoke on the subject recently with H. Jack West, MD, Swedish Cancer Institute in Seattle, Washington, and president and CEO of the Global Resource for
Advancing Cancer Education (GRACE).2
Erlotinib was licensed in 2004 for the treatment of patients with locally advanced or metastatic nonsmall cell lung cancer (NSCLC) after failure of at least one prior chemotherapy regimen. In April 2010, the FDA approved erlotinib for maintenance treatment of patients with locally advanced or metastatic NSCLC whose disease has not progressed after four cycles of platinum-based first-line chemotherapy.1
Retrospectively, Camidge said, it was evident that 10% to 15% of the treated population seemed to have the most dramatic responses. It took another five years from the time the drug was licensed to show prospectively that if selecting only for people with EGFR mutation-positive NSCLC, erlotinib or another EGFR-targeted therapy, gefitinib (Iressa, AstraZeneca), “was…actually better than chemotherapy in the first-line setting.”2
A 2011 publication of results from the SATURN trial (Sequential Tarceva in UnResectable NSCLC) underscored the power of prognostic testing to optimize outcomes
when target and target drug are in alignment. In this phase III randomized, controlled trial of 889 subjects with advanced NSCLC, subjects with EGFR mutations had a 90% reduction in the risk of death or disease progression when treated with erlotinib versus placebo (hazard ratio [HR], 0.10; P < .001).3
Compared with the years-long process resulting in matching erlotinib with its likely responders, the march from clinical research to approval of crizotinib (Xalkori, Pfizer) was lightening fast. The FDA approved the kinase inhibitor in 2011 on an accelerated basis for treatment of anaplastic lymphoma kinase (ALK)-positive patients with locally advanced or metastatic NSCLC.4 Within two years of its discovery, “dramatic responses” were being seen in the subset of lung cancer patients with a gene translocation resulting in the EML4-ALK oncogene.2 And, for the first time ever, concurrent with approval of crizotinib was approval of a companion genetic test, the Vysis ALK Break Apart FISH Probe Kit.5
Mutation Consortium (LCMC), a National Cancer Institute (NCI)-sponsored initiative made up of 14 leading cancer centers across the country. Goals of the new consortium, introduced at the 2011 American Society of Clinical Oncology (ASCO) meeting, are to identify frequencies, characteristics, and therapeutic options for genetic mutations in lung cancer. One of the LCMC’s current initiatives involves a prospective
study in which lung cancer tissue is assessed using a multiplex assay to identify and assess the 10 known driver mutations: EGFR, ALK, KRAS, HER2, BRAF, PIK3CA, AKTI, MEKI, NRAS, and MET. Early reported results (which are updated as they are acquired) showed that of the more than 800 patients on whom full mutation testing was
carried out from tumor samples, over half (54%) had single-driver mutations.7
Guidelines also reflect the changing paradigm in advanced lung cancer. In a 2011 update, the National Comprehensive Cancer Network (NCCN) guidelines called for a determination of histological subtype before conducting EGFR testing on patients with recurrent or metastatic NSCLC. EGFR testing, the authors added “…is a category 1 recommendation for adenocarcinoma, large cell, and NSCLC not otherwise specified.”8
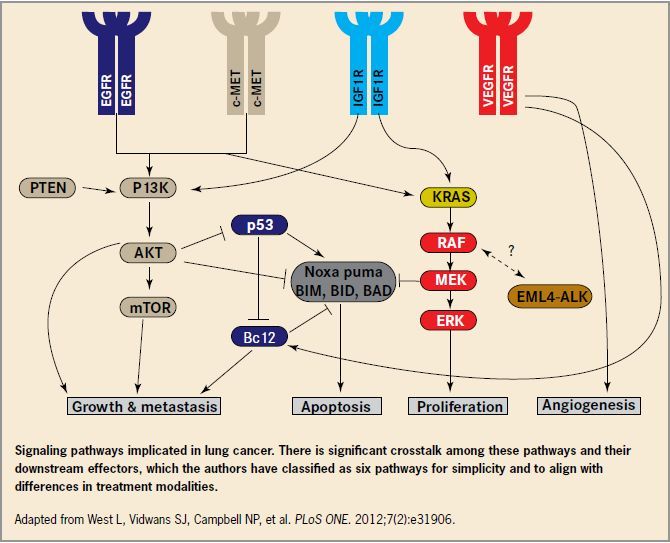
EGFR-tested patients should also undergo testing for KRAS mutations, according to the NCCN update, as both mutations are associated with intrinsic tyrosine kinase inhibitor (TKI) resistance, and thus will confer resistance to (“Launching” continued) (“Crizotinib” continued) gefitinib and erlotinib. Testing for the presence of KRAS mutations is useful in another way: Persons who are positive for KRAS mutations are unlikely to have ALK rearrangements and are thus identified as poor candidates for newly approved crizotinib, a targeted therapy for ALK-positive patients with advanced-stage NSCLC.8
References
1. Tarceva [package insert]. Farmingdale, NY: OSI Pharmaceuticals, Inc; 2011.
2. Global Resource for Advancing Cancer Education (GRACE). Dr. D. Ross Camidge on One Size Doesn’t Fit All: ALK Gene Rearrangements, ALK Inhibitors, and the Future of Lung Cancer. February 2010. Available at: http://cancergrace.org/lung/files/2010/02/dr-camidge-on-alk-inhibitors-andmolecular-oncology-transcript.pdf. Accessed March 29, 2012.
3. Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib
maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2011;(31):4113-4120.
4. Xalkori [package insert]. New York, NY: Pfizer; 2011.
5. Vysis ALK Break Apart FISH Probe Kit. Available at: http://www.abbottmolecular.com/us/products/oncology/fish/lungcancer/vysis-lsi-alk-dual-color-break-apart-rearrangementprobe. html. Accessed March 29, 2012.
6. Janne PA, Wang XF, Socinski MA, et al. Randomized phase II trials of erlotinib (E) alone or in combination with carboplatin/ paclitaxel (CP) in never or light former
smokers with advanced lung adenocarcinoma: CALGB 30406. ASCO 2010. Abstract 7503.
7. Lung Cancer Mutation Consortium (LCMC). Available at: http://www.golcmc.com. Accessed March 29, 2012.
8. National Comprehensive Cancer Network (NCCN). Updated Guidelines for Non-Small Cell Lung Cancer. Available at: http://www.nccn.org/network/business_insights/flash_updates/flash_update_information.asp?FlashID=32. Accessed March 29, 2012.
Evidence that targeted therapy is most effective when it is given to the right patient continues to mount. In one recent phase II trial (CALGB 30406) of erlotinib alone or in combination with carboplatin/ paclitaxel in never or light former smokers, patients with EGFR mutations had a significantly better response rate at 67% (P <.0001) and progression-free survival (PFS) than patients without the mutation.6 Fast-forwarding the bench-to-bedside journey of molecularly targeted agents is the Lung Cancer
