New Guideline Warns of Overstated Benefits for Medical Cannabinoids
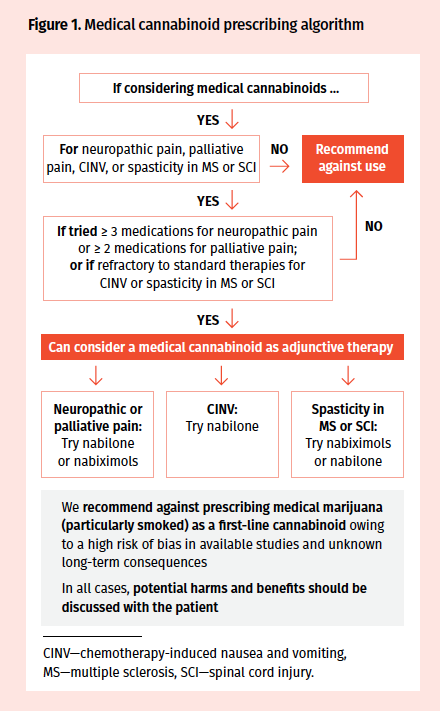
A newly released guideline for Canadian practitioners recommended against cannabinoids for most conditions, save for chronic neuropathic pain, chronic cancer-related pain, and refractory CINV and spasticity.

Mike Allan, MD, CCFP
Credit: Ross Neitz
Contention around the use of medical cannabinoids could heat up following the University of Alberta's issuance of a new guideline geared towards family physicians. Intended to simplify guidance on when to prescribe cannabinoids, it warns that the health risks of prescribing cannabinoids outweigh their benefits.
The guideline, which examined 31 systematic reviews consisting of a multitude of studies, may be unsatisfactory for some people—especially those with polarized views on medical cannabinoids, G. Michael Allan, MD, CCFP, the lead author of the guideline and the director of evidence-based medicine at the University of Alberta, said in a statement.
"For those with a fixed belief, it’s unlikely to make a change,” Allan told MD Magazine. "The target audience is the majority that is in the middle, to provide some help with the conversations for physicians who have patients that request [cannabinoids]. It provides guidance about when there isn’t any information about the benefits and adverse events when patients decide to use them."
One of the main points of disputation the investigators found is that in many cases, the size and duration of existing studies are narrow in scope.
"There are no studies for the treatment of depression. For anxiety, there is one study of 24 patients with social anxiety in which half received a single dose of cannabis derivative and scored their anxiety doing a simulated presentation. This is hardly adequate to determine if lifelong treatment of conditions like general anxiety disorders is reasonable," Allan said. "In general, we're talking about one study, and often very poorly done."
The guideline states that while the enthusiasm surrounding the use of medical marijuana is strong, there is actually limited quality research on the topic, leading to a fear that physicians may not be considering the adverse effects (AEs) of the use of these treatments.
According to the guideline, cannabinoids are strongly recommended against for the treatment of acute pain, as well as nausea and vomiting in pregnancy or hyperemesis gravidarum, due to a lack of evidence and knowledge about harms. It also recommended against using cannabinoids for headache, pain associated with rheumatologic conditions (including osteoarthritis and back pain), neuropathic pain, palliative cancer pain, general nausea and vomiting, chemotherapy-induced nausea and vomiting (CINV), and general spasticity (including in multiple sclerosis [MS] and spinal cord injury [SCI]).
The most common reason for medical marijuana use is chronic pain, varying from 58% to 84% of medical marijuana users, according to the guideline. Surveys of medical users have found that 70% or more believe medical marijuana use results in moderate or better improvement in their symptoms.
According to the review, the estimated benefit found in trial for neuropathic and cancer pain was 39% with cannabinoids compared to 30% with placebo (evidence quality [EQ], very low), for neuropathic pain alone 38% compared to 30% (EQ, very low), palliative pain 30% compared to 23% (EQ, very low). The estimated change in chronic pain scales—measured on a score of 1-10—was a decrease of 1.2-1.6 points with cannabinoids and a decrease of 0.8 points with placebo (EQ, very low).
The authors did recommend considering cannabinoids for the treatment of refractory neuropathic pain, refractory pain in patients with cancer, refractory CINV in conjunction with other therapies only after educating the patient and having a trial of standard therapies, and refractory spasticity in MS and SCI under similar conditions and only if the spasticity is persistent.
Credit: Allan GM, Ramji J, Perry D, et al.
In regard to product preference, the guideline pointed to the use of pharmaceutically developed products such as nabilone and nabiximols as the initial agents. Medical marijuana—particularly for smoking—was advised against in most scenarios due to a lack of data and a fear of bias in the existing data. The cannabinoids that were recommended for consideration were nabilone (for spasticity) and nabiximols (for pain).
“Although cannabinoids have been promoted for an array of medical conditions, the evidence base is challenged by bias and a lack of high-level research. Two large evidence synopses suggested that only 3 conditions have an adequate volume of evidence: chronic pain, nausea and vomiting, and spasticity,” the guideline states.
The guideline was developed after an in-depth review of clinical trials and a peer review conducted by 40 physicians, nurses, pharmacists, and patients. The authors did recommend long-term monitoring of medical cannabinoids in order to “further assess potential individual and societal benefits and harms.”
The guideline reported that cannabis has been used by 43% of those aged 15 and older in Canada (12% in the past 12 months), with the highest use in those aged 18 to 24 years (33%). In the United States, the most commonly reported reasons for use were recreational (53%), medicinal (11%), and both (36%). Self-reported medical use—defined as the use of dried cannabis or cannabis oil—is estimated at a rate of 15% to 19% for conditions like MS, chronic pain, and inflammatory bowel disease, as well as mental health conditions, sleep disorders, and spasticity in MS.
For the investigators, AEs were another main point of dispute. In their review, the investigators found that about 11% of patients were unable to tolerate cannabinoids compared to 3% with placebo. The most common AEs were sedation (50% with cannabinoids compared to 30% with placebo), dizziness (32% compared to 11%), and confusion (9% compared to 2%).
Allan added that both sides of the coin may end up dissatisfied with the guideline. Those in opposition of the use of cannabinoids as therapy may find it disappointing as it still considers medical cannabinoids in specific cases, while those who are in favor cannabinoids may be frustrated that the guideline is not advocating for the use of them sooner or for a broader range of conditions.
The authors concluded that: “Medical cannabinoids challenge clinicians, particularly as we attempt to provide symptom and functional improvement in patients whose conditions are refractory to other therapies. The evidence for medical cannabinoids is unfortunately sparse in many areas and very frequently downgraded by serious bias, limiting the ability to provide clear guidance. Overall, the PGC believed that medical cannabinoids are not recommended for most patients and conditions by far…When considered, there should be a discussion with patients regarding the limited benefits and more common harms, and a preferential trial of pharmaceutical cannabinoids first (over medical marijuana).”
"The next step is really in the court of the federal government and granting agencies for publicly funded trials," Allan said. "There’s no incentive for licensed producers of med marijuana to perform these trials—they're expensive, and they're not legally required for natural substances. What we should be doing is investing in large studies—we need to answer the questions. We have these small studies which don’t really inform us the same way as larger research for long periods of time would. With that, we can fill out the missing pieces."
The guideline, “Simplified Guideline for Prescribing Medical Cannabinoids in Primary Care,” was published in Canadian Family Physician.
Related Coverage >>>
Novel Pain Management Approach Uses Spearmint to Trigger Pain Relief
Buprenorphine Therapy Associated With Lower Costs, Improved Outcomes
Recovery-conscious Program Improves Surgical Recovery, Costs