Otezla Cuts Severity of Nail and Scalp Psoriasis
The severity of nail and scalp psoriasis was significantly reduced in patients who received twice-daily treatment with Otezla (apremilast, Celgene).
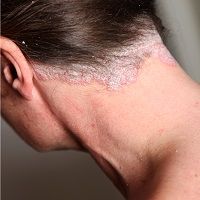
The severity of nail and scalp psoriasis was significantly reduced in patients who received twice-daily treatment with Otezla (apremilast, Celgene).
To assess the efficacy of Otezla in nail and scalp psoriasis, researchers randomly assigned a cohort of 1,255 patients afflicted with moderate-to-severe psoriasis to receive either Otezla 30 mg twice daily or placebo in the phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) 1 and 2 trials.
During week 16, the placebo patient group was switched to apremilast through week 32 and randomly selected for the withdrawal phase to week 52.
At baseline, 66.1% and 64.7% of patients had nail psoriasis and 66.7% and 65.5% had moderate-to-very severe scalp psoriasis in ESTEEM 1 and 2. Study results indicated a significant improvement in the NAPSO score within patients treated with apremilast at week 16 compared with the placebo group.
Researchers found, “although the nails and scalp account for a small percentage of the body surface area, psoriasis in these areas can have a disproportionate effect on a patient’s physical and psychosocial function.”
According to the study results, “Apremilast, a novel oral therapeutic for patients with moderate-to-severe plaque psoriasis, is an effective option for patients with nail and/or scalp involvement,” concluded the authors.