Psoriatic Arthritis: A Disease in Full
The concept of psoriatic arthritis (PsA) has evolved from that of a relatively obscure mild joint disease to that of a severe inflammatory arthritis involving bones, joints, and the surrounding tissue.
ABSTRACT: The concept of psoriatic arthritis (PsA) has evolved from that of a relatively obscure mild joint disease to that of a severe inflammatory arthritis involving bones, joints, and the surrounding tissue. Patients are at increased risk for several comorbidities, including obesity and metabolic syndrome. PsA is an aggressive disease that often leads to peripheral joint damage, associated functional decline, and impaired quality of life. The variable early symptoms may create considerable diagnostic uncertainty. Several screening instruments have been developed. The large number of domains that may be affected in a single patient with PsA remains a major challenge; development of comprehensive instruments is under way. Biomarker development in PsA is in its early stages. The role of imaging studies in patient assessment is expanding, especially ultrasonography and MRI. (J Musculoskel Med. 2011;28:95-101)
The concept of psoriatic arthritis (PsA) has evolved considerably over the past decade from that of a relatively obscure, mild joint disease to that of a prevalent, complex, potentially disabling musculoskeletal syndrome. In addition, patients with psoriasis or PsA are now recognized to be at increased risk for a spectrum of comorbidities, including obesity, metabolic syndrome, diabetes mellitus, and cardiovascular disease.
The multifaceted nature of PsA may be observed in the heterogeneous clinical presentations elegantly described by Moll and Wright.1 It also can be seen in the myriad forms of tissue inflammation that involve not only the skin and peripheral joints but also entheses, nails, tendons, and structures in the axial skeleton. In addition, studies have expanded the spectrum of target tissues in psoriatic disease to include the eyes, gut, vasculature, and endocrine system.2
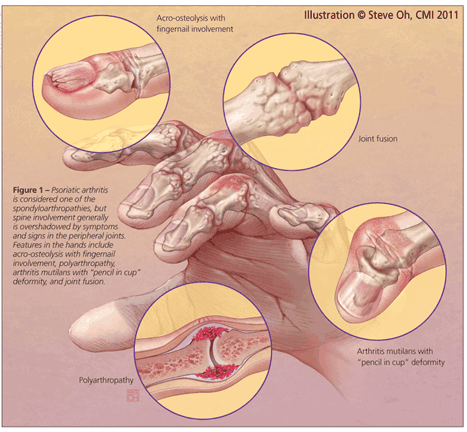
Although PsA is considered a member of the spondyloarthropathies, spine involvement generally is overshadowed by symptoms and signs in the peripheral joints (Figure 1). In fact, a growing body of evidence has demonstrated that PsA is an aggressive disease that often leads to peripheral joint damage, associated functional decline, and impaired quality of life.3,4 With this new evidence, the importance of early diagnosis and initiation of aggressive treatment has been emphasized, although data to support the validity of this approach are not yet available.
Randomized trials of PsA conducted over the past several years have included additional end points that have provided insights into the effectiveness of biologic agents for the management of various domains, such as dactylitis and enthesitis. In addition, recent data derived from a combination of clinical trials and registries have resulted in a better understanding of the efficacy and durability of methotrexate (MTX) in PsA.
This 2-part article provides an update on the diagnosis and management of PsA. In this first part, we discuss advances in the assessment of early and established PsA and the efforts under way to develop accurate and reliable biomarkers, as well as the expanding role of imaging studies in diagnosis and assessment of patients with psoriasis and PsA. The second part, to appear in an upcoming issue of this journal, reviews the latest studies that address the efficacy of MTX and biologic agents for patients with psoriatic disease and provides a brief overview of new agents in the pipeline.
SCREENING INSTRUMENTS IN THE OUTPATIENT SETTING
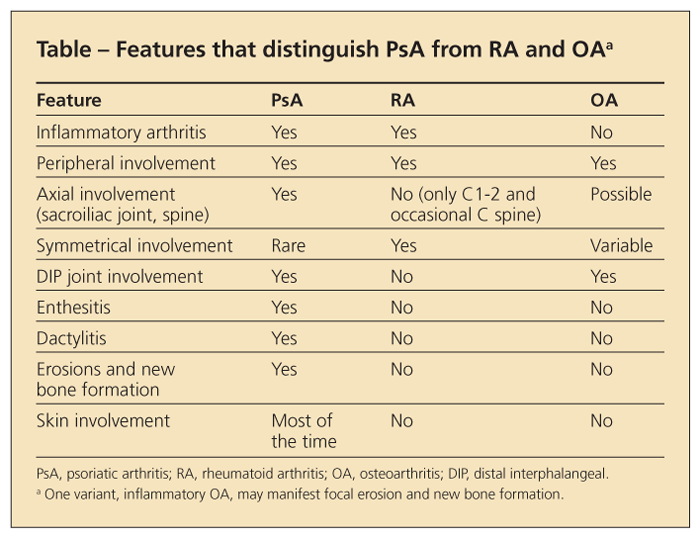
The extremely variable early symptoms of PsA may pose considerable diagnostic challenges (Table). Given that skin lesions precede the development of PsA by about 10 years in most patients,5 dermatologists often are in the best position to detect early joint involvement.6
With this in mind, several screening instruments have been developed for identification of patients who have psoriasis with subclinical musculoskeletal disease, including the Psoriatic Arthritis Screening and Evaluation (PASE), Toronto Psoriatic Arthritis Screen (ToPAS), and Psoriasis Epidemiology Screening Tool (PEST) questionnaires. Dominguez and associates7 recently reviewed the specific details of these screening instruments.
The PASE tool can distinguish between PsA and noninflammatory arthritis (osteoarthritis) and is useful in distinguishing among more severe subtypes of PsA.8 The ToPAS questionnaire was designed as a screening tool for PsA regardless of whether a patient has psoriasis; it demonstrated high sensitivity and specificity for identification of PsA in various patient groups (in general dermatology, rheumatology, and family medicine clinics).9 The PEST instrument was developed by consolidating 2 older existing questionnaires, the Psoriatic Arthritis Questionnaire (PAQ) and the Alenius modification of PAQ; it showed very good sensitivity (0.97) but poor specificity (0.79).
Although there is no consensus to date as to which screening tool is the most useful, studies are in progress to determine the most appropriate setting for each. An important unmet need for the identification of early PsA would be addressed if a patient with psoriasis in the dermatologist’s reception area was given a simple questionnaire to complete that could be scored rapidly and reliably and that accurately detects early arthritis.
OUTCOME MEASURES
The large number of domains (skin, peripheral joints, axial skeleton, dactylitis, enthesitis) that may be affected in a single patient with PsA remains a major challenge. Attempts to develop comprehensive instruments to assess these domains are under way and in various stages of validation.
Major barriers to comprehensive assessment tools may consist of complex measures designed to encompass the wide array of clinical manifestations of PsA; the measures may be cumbersome and unfeasible in the clinical setting. In addition, the presence of multiple clinical manifestations that may track independently (eg, skin and joint inflammation) would not be captured in an overly simplified assessment tool.
A comprehensive list of domains of assessment in PsA published in 2008 illustrates the complexity.10 It includes not only assessment of joints, skin, and nails but also patient and physician global assessment and assessment for fatigue, enthesitis, dactylitis, acute phase reactants, and imaging modalities.
The 66/68-joint count is recommended for clinical trials, but the 28-joint Disease Activity Score is more feasible in clinical practice. However, one study showed that the global health assessment in this measure is bidimensional and reflects activity in both the skin and joints, a finding that limits its validity as a measure of joint inflammation in PsA.11 In another study, 3 joint counts (28, 32, and 36) compared favorably with the 66/68-joint count when analyzed retrospectively on a clinical trial dataset.12 However, the abbreviated counts have a major shortcoming-the possibility of underestimating oligoarticular disease because the feet are not part of the calculation.
Efforts are under way to develop a composite measure of disease activity that would help clinicians make treatment decisions in PsA. Using a factor analysis approach, Nell-Duxneuner and colleagues13 developed the Disease Activity index for PSoriatic Arthritis, a measure that includes the Visual Analogue Scale (VAS) of patient global disease activity assessment, VAS pain assessment, 66/68–swollen and –tender joint count, and C-reactive protein (CRP) level. Skin assessment was excluded because it did not reach statistical significance. Another measure, the Composite Psoriatic Disease Activity Index (CPDAI), addresses 5 domains (skin, peripheral joints, axial disease, dactylitis, and enthesitis) and grades them as mild, moderate, or severe.
Both assessment tools were effective in predicting joint treatment response to etanercept in a clinical trial dataset, but the CPDAI provided additional information regarding activity and response in the skin, entheses, and dactylitic joints.14 More studies of these and other composite indices are under way to determine which of them will be most appropriate in the clinical setting.
BIOMARKERS
Biomarker development in PsA is in its early stages. Biomarkers could assist in identification of patients with psoriasis at risk for arthritis (susceptibility), help predict treatment response (response), serve as surrogates for radiographic outcomes (surrogate), and help predict which patients are at risk for a more severe course (severity). Some classes of biomarkers may be of benefit in PsA, but to date no biomarkers have been properly validated.
A genetic marker that can serve as a measure of severity, such as anti–cyclic citrullinated peptide antibodies in rheumatoid arthritis (RA), has not been identified in patients with PsA. However, genome-wide association studies have found genes of interest (MICA-A9, TRAF3IP2) that are associated with PsA but not with psoriasis.15,16 An elevated CRP level denotes a subpopulation of patients with PsA receiving MTX who are at risk for greater radiographic progression, but this soluble marker is not predictive of radiographic progression in patients who are receiving anti–tumor necrosis factor α (anti–TNF-α) agents.17 Higher serum levels of interleukin (IL)-6, soluble receptors of IL-2, IL-1 receptor antagonists (IL-1ra), and IL-10 were found in patients with PsA than in healthy volunteers, but only the erythrocyte sedimentation rate and IL-1ra were associated with joint severity.18
TNF-α, matrix metalloproteinase (MMP)-2, vascular endothelial growth factor, and E-selectin were measured in the sera and in the lesional skin of 11 patients with PsA, before and after treatment with infliximab.19 A significant decrease of MMP-9 and MMP-2 levels in the sera was associated with clinical improvement in the joint domain; a decrease of MMP-9, TNF-α, and E-selectin levels was associated with clinical improvement in skin disease.
Recent evidence indicates that the TH17 pathway is of central importance in PsA; TH17 cells in peripheral blood and in the joint release increased quantities of IL-17 in cell culture compared with cells from controls.20 In other studies, osteoclast precursors were noted to be elevated in patients with PsA who manifest a higher level of radiographic damage, and they decline rapidly after anti–TNF-α therapy.21,22
The introduction of microarray techniques has opened new avenues for biomarker discovery. Peripheral blood mononuclear cells from 19 patients with PsA were analyzed in an attempt to better characterize PsA phenotype using gene expression profiling.23 The pattern of expression was altered significantly for 313 genes; the large majority showed down-regulation. Those genes tended to cluster to certain chromosomal regions, including those containing the psoriasis susceptibility loci PSORS1 and PSORS2. With the use of 48 RA control samples, all patients who had RA or PsA were classified correctly with the combination of 2 genes, MAP3K3 and a calcium channel gene, CACNA1S.
A variety of down-regulated genes were identified in microarray studies of a different set of patients.24 Two lymphocyte-specific genes discriminated patients with PsA from controls, and many suppressed genes fell in the CD40-signaling or T-cell–signaling pathways. Adequately powered studies that examine the various types of biomarkers in subjects enrolled in well-designed clinical trials and observational studies will provide important insights into the role of biomarkers in PsA.
IMAGINGPlain x-ray films
Plain films of affected joints and of the anteroposterior pelvis traditionally have been obtained in patients who are undergoing evaluation for PsA. Their advantage comes from their easy access, low cost, and relatively good sensitivity in detecting bone erosions. At the same time, they have several important limitations that arise from the heterogeneity of tissue involvement in PsA, which includes not only bone and joints but also tendons, ligaments, and soft tissue. Plain x-ray films cannot identify inflammation or damage of the ligamentous structures or attachment sites (enthesitis, dactylitis) or reveal small erosions in early disease or bone marrow edema.
Ultrasonography (US)
This modality is of particular interest in PsA because it provides the operator with the ability to detect early joint and soft tissue inflammation and bone erosions. The addition of a Doppler signal provides more information about vascularity observed in both synovial and entheseal inflammation.
In a case-control study of lower limbs in 30 patients who had psoriasis but not arthritis and 30 matched controls, the Glasgow Ultrasound Enthesitis Scoring System (GUESS) score was significantly higher in patients with PsA than in controls.25 Of note, the GUESS score correlated with age, body mass index, and waist circumference but not with duration or severity of arthritis. The high prevalence of subclinical joint involvement is in accordance with findings on MRI and scintigraphy reported in similar populations.26,27
In a comparative study of musculoskeletal US findings in 25 patients with RA and 25 patients with PsA conducted by Fourni and colleagues,28 US was more sensitive than standard radiography for the detection of joint erosions. In addition, synovial abnormalities and joint erosions were observed consistently in the patients with RA.
Of note, extrasynovial changes were found in 84% of the fingers with PsA versus none of the fingers with RA; 60% of the fingers with PsA had synovial changes. The extrasynovial changes included juxta-articular periosteal reaction, capsular enthesophytes, and enthesopathy at the attachment of the deep flexor tendon on the distal phalanx.
Similar to the findings noted in some patients with psoriasis but not arthritis, thickening of the soft tissue (tendon ligaments) suggestive of inflammation was observed in the patients with PsA. Another interesting finding was the presence of a Doppler signal at the base of the nail, which suggests increased periungual vascularity. These findings underscore the concept that like RA, PsA can involve the synovium, but it also has characteristic pathologies centered around entheses and soft tissue that typically are not observed in the rheumatoid joint.
Wiell and associates29 assessed US changes in small joints and compared them with MRI, radiographic, and clinical findings in 15 patients with PsA, 5 with RA, and 5 healthy controls. US revealed more frequent synovitis in the RA group than in the PsA group in the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints but less frequent bone erosions in the PIP joints. Also, distal interphalangeal (DIP) joint bone changes were found exclusively in patients with PsA. In addition, bone proliferations were more common and tenosynovitis was less frequent in patients with PsA than in those with RA.
This was the first study to compare MRI, US, and clinical examinations directly in patients with PsA and RA; the findings are concordant with those in previous studies.30-33 US and MRI were more sensitive in the detection of inflammatory and destructive changes than were radiography and clinical examination. A high interobserver agreement (85% to 100%) between US and MRI was noted for destructive changes, but a more moderate agreement (73% to 100%) was observed for the inflammatory pathologies.
The high concordance rate is encouraging, particularly in light of recent concerns centered on a lack of interreader reliability. US most likely will take on an increasingly important role in the evaluation and management of PsA. However, additional studies are required to better understand the potential pitfalls and to develop teaching modules that will produce highly skilled operators.
MRI
This modality has distinct advantages for the imaging of psoriatic joints because of its ability to capture high-resolution images of tenosynovitis, bone marrow edema, and early erosions.34 However, MRI has limitations, including high cost and variable reproducibility. The development of the Rheumatoid Arthritis Magnetic Resonance Imaging Score system by the Outcome Measures in Rheumatology Clinical Trials35 has resulted in improved interobserver reliability for MRI parameters.
The PsA MRI scoring system (PsAMRIS) was developed from the same model. MRI definitions of key PsA pathologies were agreed on by consensus and subjected to a new exercise.36 Subsequent scores revealed moderate to high reliability for most features but still showed poor reliability for periarticular inflammation. Change scores that reflected inflammatory activity also exhibited moderate to good reliability in the longitudinal exercise, in spite of very little absolute change in MRI synovitis or tenosynovitis.
MRI scoring for the small joints of the hands and feet remains a challenge. Detecting pathology in these very small joints can be difficult, increasing the likelihood of errors. Also, the fat saturation can be inhomogeneous, leading to false-positive bone marrow edema signals.
The preliminary PsAMRIS for PsA of the hands was developed in an attempt to standardize the scoring and overcome the above shortcomings.37 The authors provide MRI definitions for characteristic components of PsA (synovitis, tenosynovitis, periarticular inflammation, bone edema, and bone erosion and proliferation) and useful scoring sheets for MCP, PIP, and DIP joint regions, which need to be validated in clinical trials.
An important question for clinicians is whether MRI can differentiate PsA from RA. To address this question, Marzo-Ortega and colleagues38 used dynamic contrast-enhanced MRI to scan the swollen MCP joints of 10 patients who had PsA and 10 patients who had RA. Synovitis, tenosynovitis, periarticular bone erosion, and bone edema were scored.
Significantly more synovitis was seen in the patients with RA than in those with PsA, but there were no significant differences between the groups in the number or size of determined erosions on MRI. Also, the pattern of enhancement was mainly synovial in both groups, with more striking extracapsular enhancement in the PsA group.
A proof of concept study of whole-body MRI (WB-MRI) was performed recently on 30 patients with PsA to assess areas of inflammation not accessible to clinical examination (Figure 2).39 MRI detected 80% more enthesitis sites than the clinical examination; there was concordance between the clinical examination and the MRI scan in only 10% of cases. Similarly, MRI detected 57% more
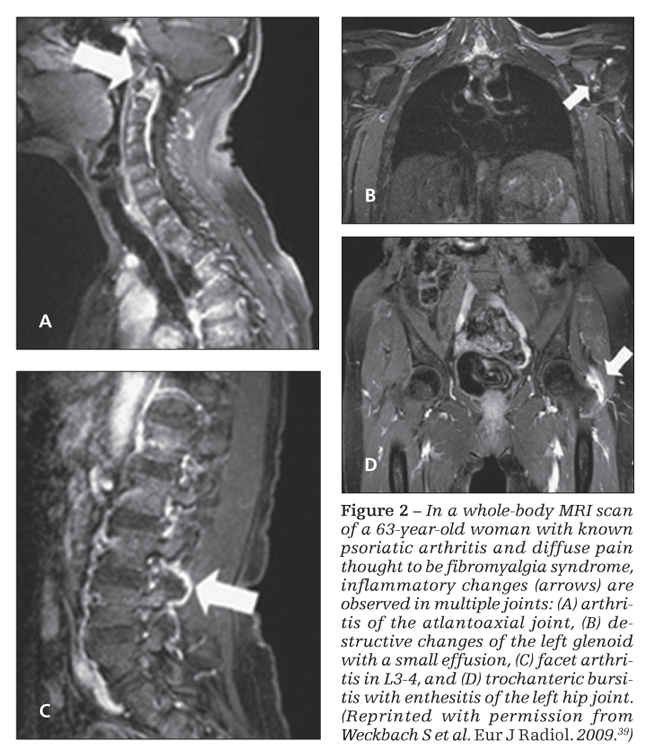
synovitis, with a better clinical correlation: in 43% of patients, the clinical examination paralleled the MRI findings. Even more interesting, the WB-MRI findings changed the therapeutic decision in 73% of patients, and TNF-α inhibitor therapy was started for 62% of patients.
A range of imaging modalities can help the clinician in diagnosis, disease activity assessment, and implementation of anti-inflammatory therapies. Given the lack of diagnostic biomarkers, the diagnosis of PsA is largely based on both clinical and imaging assessments. In the right clinical context, radiological evidence of bone ankylosis coexistent with erosions in a pattern characteristic of PsA strongly supports the diagnosis. Similarly, bone marrow edema (detected by MRI) and tenosynovitis (detected by MRI or US) have diagnostic utility, although these findings are not specific, because similar findings-generally of lesser magnitude and extent-may be observed in RA.
Imaging also may be used to detect disease progression. In this regard, MRI and US are far superior to plain radiography but with the above-mentioned limitations. Detection of bone pathology typically is useful in guiding treatment decisions. A finding of a new erosion, enthesitis, or bone marrow edema reflects active disease and as such often prompts initiation or alteration of treatment.
References
1. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55-78.
2. Ritchlin C. Psoriatic disease-from skin to bone. Nat Clin Pract Rheumatol. 2007;3:698-706.
3. Turkiewicz AM, Moreland LW. Psoriatic arthritis: current concepts on pathogenesis-oriented therapeutic options. Arthritis Rheum. 2007;56:1051-1066.
4. McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (Oxford). 2003;42:778-783.
5. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
6. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748; quiz 749-750.
7. Dominguez P, Gladman DD, Helliwell P, et al. Development of screening tools to identify psoriatic arthritis. Curr Rheumatol Rep. 2010;12:295-299.
8. Husni ME, Meyer KH, Cohen DS, et al. The PASE questionnaire: pilot-testing a psoriatic arthritis screening and evaluation tool. J Am Acad Dermatol. 2007;57:581-587.
9. Gladman DD, Schentag CT, Tom BD, et al. Development and initial validation of a screening questionnaire for psoriatic arthritis: the Toronto Psoriatic Arthritis Screen (ToPAS). Ann Rheum Dis. 2009;68:497-501.
10. Mease PJ. Assessment tools in psoriatic arthritis. J Rheumatol. 2008;35:1426-1430.
11. Leeb BF, Andel I, Sautner J, et al. The Disease Activity Score in 28 joints in rheumatoid arthritis and psoriatic arthritis patients. Arthritis Rheum. 2007;57:256-260.
12. Englbrecht M, Wang Y, Ronneberger M, et al. Measuring joint involvement in polyarticular psoriatic arthritis: an introduction of alternatives. Arthritis Care Res (Hoboken). 2010;62:977-983.
13. Nell-Duxneuner VP, Stamm TA, Machold KP, et al. Evaluation of the appropriateness of composite disease activity measures for assessment of psoriatic arthritis. Ann Rheum Dis. 2010;69:546-549.
14. FitzGerald O, Helliwell P, Mumtaz A, et al. Application of composite disease activity scores in psoriatic arthritis to the PRESTA dataset. Arthritis Rheum. 2010;62:S214.
15. Gonzalez S, Martinez-Borra J, Torre-Alonso JC, et al. The MICA-A9 triplet repeat polymorphism in the transmembrane region confers additional susceptibility to the development of psoriatic arthritis and is independent of the association of Cw*0602 in psoriasis. Arthritis Rheum. 1999;42:1010-1016.
16. Hffmeier U, Uebe S, Ekici AB, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet. 2010;42:996-999.
17. Gladman DD, Mease PJ, Healy P, et al. Outcome measures in psoriatic arthritis. J Rheumatol. 2007;34:1159-1166.
18. Elkayam O, Yaron I, Shirazi I, et al. Serum levels of IL-10, IL-6, IL-1ra, and sIL-2R in patients with psoriatic arthritis. Rheumatol Int. 2000;19:101-105.
19. Cordiali-Fei P, Trento E, D’Agosto G, et al. Effective therapy with anti-TNF-alpha in patients with psoriatic arthritis is associated with decreased levels of metalloproteinases and angiogenic cytokines in the sera and skin lesions. Ann N Y Acad Sci. 2007;1110:578-589.
20. Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876-2885.
21. Ritchlin CT, Haas-Smith SA, Li P, et al. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821-831.
22. Anandarajah AP, Schwarz EM, Totterman S, et al. The effect of etanercept on osteoclast precursor frequency and enhancing bone marrow oedema in patients with psoriatic arthritis. Ann Rheum Dis. 2008;67:296-301.
23. Batliwalla FM, Li W, Ritchlin CT, et al. Microarray analyses of peripheral blood cells identifies unique gene expression signature in psoriatic arthritis. Mol Med. 2005;11:21-29.
24. Stoeckman AK, Baechler EC, Ortmann WA, et al. A distinct inflammatory gene expression profile in patients with psoriatic arthritis. Genes Immun. 2006;7:583-591.
25. Gisondi P, Tinazzi I, El-Dalati G, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26-30.
26. Namey TC, Rosenthall L. Periarticular uptake of 99mtechnetium diphosphonate in psoriatics: correlation with cutaneous activity. Arthritis Rheum. 1976;19:607-612.
27. Offidani A, Cellini A, Valeri G, Giovagnoni A. Subclinical joint involvement in psoriasis: magnetic resonance imaging and X-ray findings. Acta Derm Venereol. 1998;78:463-465.
28. Fourni B, Margarit-Coll N, Champetier de Ribes TL, et al. Extrasynovial ultrasound abnormalities in the psoriatic finger: prospective comparative power-Doppler study versus rheumatoid arthritis. Joint Bone Spine. 2006;73:527-531.
29. Wiell C, Szkudlarek M, Hasselquist M, et al. Ultrasonography, magnetic resonance imaging, radiography, and clinical assessment of inflammatory and destructive changes in fingers and toes of patients with psoriatic arthritis [published correction appears in Arthritis Res Ther. 2008;10:402]. Arthritis Res Ther. 2007;9:R119.
30. Dhn UM, Ejbjerg BJ, Court-Payen M, et al. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther. 2006;8:R110.
31. Szkudlarek M, Klarlund M, Narvestad E, et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther. 2006;8:R52.
32. Szkudlarek M, Narvestad E, Klarlund M, et al. Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum. 2004;50:2103-2112.
33. Wakefield RJ, Emery P, Veale D. Ultrasonography and psoriatic arthritis. J Rheumatol. 2000;27:1564-1565.
34. McQueen FM, Dalbeth N, Doyle A. MRI in psoriatic arthritis: insights into pathogenesis and treatment response. Curr Rheumatol Rep. 2008;10:303-310.
35. stergaard M, Peterfy C, Conaghan P, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies: core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system [published correction appears in J Rheumatol. 2004;31:198]. J Rheumatol. 2003;30:1385-1386.
36. McQueen F, Lassere M, Duer-Jensen A, et al. Testing an OMERACT MRI scoring system for peripheral psoriatic arthritis in cross-sectional and longitudinal settings. J Rheumatol. 2009;36:1811-1815.
37. stergaard M, McQueen F, Wiell C, et al. The OMERACT psoriatic arthritis magnetic resonance imaging scoring system (PsAMRIS): definitions of key pathologies, suggested MRI sequences, and preliminary scoring system for PsA Hands [published correction appears in J Rheumatol. 2010;37:2198]. J Rheumatol. 2009;36:1816-1824.
38. Marzo-Ortega H, Tanner SF, Rhodes LA, et al. Magnetic resonance imaging in the assessment of metacarpophalangeal joint disease in early psoriatic and rheumatoid arthritis [published correction appears in Scand J Rheumatol. 2010;39:527]. Scand J Rheumatol. 2009;38:79-83.
39. Weckbach S, Schewe S, Michaely HJ, et al. Whole-body MR imaging in psoriatic arthritis: additional value for therapeutic decision making. Eur J Radiol. 2009 Jul 24; [Epub ahead of print].