Recognizing Rheumatologic Aspects of Cocaine Abuse
Thirty million Americans have used cocaine, making the United States the world’s largest consumer of the substance; there are about 5 million current users.
ABSTRACT: Cocaine has been implicated in many rheumatologic conditions. Recognition of these syndromes is important for appropriate diagnosis and management because they often are confused and misdiagnosis results. The principal effects of cocaine are seen in the CNS and the corticomesolimbic dopamine reward pathway. Whether cocaine itself or in combination with host proteins is immunogenic and whether such a phenomenon has clinical relevance is unclear. Levamisole was recently found to contaminate up to 70% of cocaine samples; it has been postulated to cause various cutaneous lesions. Cocaine-induced midline destructive lesions have been associated with cocaine. Cocaine may have deleterious effects on the cardiovascular system. Management strategies include making the diagnosis, gaining knowledge of the disorders that cocaine can mimic, and testing for cocaine and levamisole contaminant. (J Musculoskel Med. 2012;29:34-40)
Thirty million Americans have used cocaine, making the United States the world’s largest consumer of the substance; there are about 5 million current users.1 More than 70% of cocaine samples have been contaminated with levamisole, an immunomodulatory agent associated with multiple medical complications.
Cocaine, especially contaminated cocaine, has been implicated in many rheumatologic conditions, such as cutaneous vasculitis, midline granulomatous lesions, cerebral vasculitis, and coronary aneurysms. Prompt recognition of these syndromes is important for appropriate diagnosis and management because they often are confused and misdiagnosis results. In this article, we describe these syndromes and provide clues that help with early diagnosis and appropriate management of cocaine-induced rheumatologic mimics.
Background and History
Cocaine is a tropane ester alkaloid found in the leaves of Erythroxylum coca, a bush that grows in the Andes Mountains in South America.2 Its stimulant properties have been known to mankind for more than 2000 years. Cocaine has remained a vital part of the religious beliefs of the Andean peoples of Peru, Bolivia, Ecuador, Colombia, and northern Argentina and Chile from the pre-Inca period through the present.
Key events in the history of cocaine include the following:
• In 1596, cocaine was first used for medicinal purposes by a Spanish physician.
• In 1860, cocaine was isolated from the coca leaf by a German graduate student, Albert Niemann, who also is credited with coining the word “cocaine.”
• In 1884, Freud first reported the major effects of cocaine as a stimulant against hunger and fatigue, its efficacy in high-altitude sickness, and its effects as a local anesthetic and hemostatic agent.
• In 1914, the Harrison Narcotic Act designated cocaine as a prescription drug and placed it under Schedule II of the Controlled Substances Act.
A Global Menace
There are a reported 14 million cocaine users worldwide.1 In 2002 and 2003, more than 5.9 million persons (2.5%) aged 12 years or older admitted to using cocaine within the previous year. In the United States, cocaine use rates ranged from 1.6% in Idaho to 3.9% in Colorado. The annual consumption of cocaine worldwide is about 600 tons; the United States consumes about 300 tons.
Mechanism of Action
The principal effects of cocaine, a monoamine neurotransmitter reuptake inhibitor, are seen in the CNS (vasoconstriction, tachycardia, hypertension, arrhythmias, seizures, mydriasis, hyperglycemia, and hyperthermia) and the corticomesolimbic dopamine reward pathway (acute use of cocaine elevates dopamine levels and causes euphoria; long-term use causes dopamine depletion, leading to dysphoria and craving). Cocaine also blocks sodium-gated ion channels, causing cardiac arrhythmias and local anesthetic effects. Cocaine-induced arterial spasm may cause endothelial injury and promote platelet aggregation and adherence.3
Immunogenicity
Whether cocaine itself or in combination with host proteins is immunogenic and whether such a phenomenon has clinical relevance are unclear. According to Deng and associates,4 however, cocaine covalently modifies proteins through a reaction where the methyl ester acylates the ε-amino group of lysine residues. Plasma samples from long-term (14 ± 3 years) cocaine users and controls were analyzed for anti-cocaine antibodies; they were detected in 2 of 7 cocaine users and in none of the controls. The authors concluded that modification of endogenous proteins by the benzoylecgonine moiety of cocaine could produce an immunogen that might explain some of the drug’s autoimmune effects.
Additional studies in preclinical models also have demonstrated nonspecific immunological effects of cocaine on both innate and adaptive immune responses.5 However, the clinical implications of these phenomena remain unclear.
Adulterants in Cocaine
Cutting, or “stepping on,” cocaine is common, and compounds are used (eg, novocain, ephedrine, caffeine, methylphenidate, and inactive sugars) that simulate ingestion effects. The recent finding that levamisole (a synthetic imidazothiazole derivative) contaminates up to 70% of cocaine samples probably is the result of the effort to increase the bulk of the cocaine because of the high cost and demand for the drug. Levamisole is thought to enhance the euphoric effects of cocaine because of inhibition of dopamine reuptake in the presynaptic neurons.
Levamisole was first reported as a contaminant by the US Drug Enforcement Administration in 2008.6 It is a powerful immunomodulatory agent that has been used as adjuvant therapy for adenocarcinoma of the colon, in relapsing nephrotic syndrome in children, and as a slow-acting antirheumatic medication for patients with rheumatoid arthritis (RA). Levamisole may restore depressed immune function and stimulate antibodies by increasing T-cell activation and proliferation and increasing neutrophil mobility, adherence, and chemotaxis.7
Levamisole was withdrawn from the market in 2000 because of adverse effects, including neutropenia, cutaneous ulcers/vasculitis, convulsions, hypogammaglobulinemia, and arthralgias. It currently is used only in veterinary medicine as an antihelminthic medication. Levamisole may be detected in urine and blood only by gas chromatography and mass spectrometry within 48 hours of contaminated cocaine use.8
Levamisole has been postulated to cause various cutaneous lesions, such as nodules, drug eruptions, palpable purpura, and ulcers. The first case of levamisole-induced cutaneous vasculitis was reported in 1978 in a 59-year-old woman who was receiving levamisole 150 mg/d 3 times a week for 3 months for breast cancer.9 Fever, neutropenia, and rash developed, and a skin biopsy revealed vasculopathy. The symptoms resolved within 2 weeks after discontinuation of the drug and a short course of prednisone.
These lesions, which have a peculiar predilection for the ear lobules and cheeks, also have been observed when levamisole has been used as adjuvant therapy for pediatric nephritic syndrome and as a slow-acting antirheumatic agent for RA. They have been reported to appear between 12 and 44 months after the introduction of levamisole.7 Patients also have concurrent neutropenia and autoantibodies.
In our opinion, levamisole is the likely culprit in most cases of cocaine-related cutaneous lesions. As mentioned above, the immunomodulatory effects of levamisole probably contribute to the clinical features.
Detection of Cocaine and Metabolites
Cocaine is about 90% plasma-bound; the plasma half-life is short, ranging from 0.5 to 1.5 hours. About 90% of a cocaine dose is metabolized to its major metabolites-ecgonine methyl ester and benzoylecgonine.
Cocaine may be detected in urine up to 2 to 4 days after consumption and its metabolites for 6 to 14 days after illicit drug use.10 It may be detected in body fluids and tissues, such as urine, hair, saliva, sweat, nails, pericardial fluid, vitreous humor, and organs (brain/muscle/bones). Urine drug screens are performed frequently because of their convenience, low invasiveness, and accuracy and the higher concentrations of the drug in urine than in blood or plasma.
The screening test is done by immunoassay and confirmed with gas chromatography-mass spectrometry.11 Hair analysis represents an alternative for cocaine testing because the drug may be detected for weeks to months after use. Oral fluid analysis detects non–protein-bound cocaine and may detect the drug up to 17 hours after the last dose.10
Cocaine and Rheumatologic Mimics
Cocaine (and its contaminants) may present in ways that mimic a number of rheumatologic conditions and cause diagnostic confusion. Conditions that have been associated with cocaine include the following:
FIGURE 1
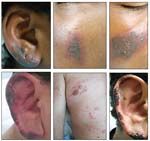
Cocaine-related cutaneous lesions are shown in these photographs. (Reproduced with permission from Chung C et al.
J Am Acad Dermatol
. 2011.
27
© 2010. American Academy of Dermatology.)
• Cutaneous lesions. Cocaine always should be considered as a possible cause of unexplained skin lesions in vulnerable patient populations (Table 1). The most characteristic rash associated with cocaine is that of cutaneous necrosis, especially when it involves the face, including the ears and nose (Figure).
TABLE 1

Case reports and series of cocaine-induced cutaneous lesions
a
A similar rash may be seen in patients with cryoprotein disorders, coagulopathies, systemic vasculitis, and frostbite, as well as other conditions. Epidemiologically, however, cocaine and levamisole exposure ranks high in the differential diagnosis (Table 2).
Warfarin-induced skin necrosis may be confused with cocaine-related cutaneous lesions. This entity may be seen in obese women, typically those who are protein C– or protein S–deficient, within 1 to 10 days of warfarin exposure. Warfarin-induced skin necrosis often involves fatty areas of the body, such as the breasts, buttocks, and thighs. The lesions consist of bullae and necrosis; skin biopsy reveals bland thrombi.
TABLE 2
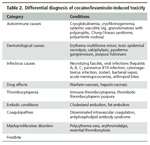
Differential diagnosis of cocaine/levamisole-induced toxicity
Heparin-induced cutaneous necrosis, a rare manifestation of heparin-induced thrombocytopenia, also causes tender, noninflamed lesions on the abdomen and extremities. It usually is seen 4 to 10 days after initial exposure to heparin. Skin biopsy shows bland thrombi.12 The pathophysiology of some of these lesions is unclear because they are similar to the skin lesions ascribed to levamisole and thus may be immune-mediated. Alternatively, cocaine itself may play a role because of its vasoconstrictive effects on the small vessels, especially in peripheral areas such as the ears.13
• Midline granulomatous lesions. Cocaine-induced midline destructive lesions (CIMDL), a syndrome of extensive destruction of the nose, palate, and midline structures, recently was associated with cocaine. A 2004 study tested human neutrophil elastase (HNE) antineutrophil cytoplasmic antibodies (ANCAs) reactivity in 25 patients with CIMDL.14 The controls consisted of 604 consecutive patients who had granulomatosis with polyangiitis (GPA) (formerly known as Wegener granulomatosis), microscopic polyangiitis (MPA), or another rheumatologic disorder and healthy volunteers. HNE ANCAs were detected by indirect immunofluorescence and direct and capture enzyme-linked immunosorbent assay.
In patients who had CIMDL, HNE ANCAs were detectable in 84% by 1 assay, in 68% by 2 assays, and in 36% by 3 assays. Of HNE ANCA–positive CIMDL sera, 57% were positive for proteinase 3 (PR3) ANCA by at least 1 assay. Only 1.3% of controls reacted with HNE in at least 1 assay, 0.5% in 2 assays, and 0.16% in 3 assays. The sera of patients with GPA or MPA and healthy volunteers were negative for HNE ANCAs. The authors concluded that optimal sensitivity for HNE ANCAs requires multimodality testing; HNE ANCAs are frequent in CIMDL but not in classic ANCA-associated vasculitis.
The mechanism of action of cocaine in such cases may be related to the production of HNE ANCAs. HNE and PR3 are part of the chymotrypsin family of serine proteinases. HNE ANCAs may activate primed neutrophils, modify clearance of apoptotic cells, and enhance or perturb neutrophil apoptosis. A high frequency of apoptotic cells has been described in CIMDL.
Cocaine has a vasoconstrictive and irritant effect that may lead to inflammation, ulceration, erosion, and disintegration of the nasal septum-with bleeding and perforation-leading to a saddle nose deformity. There also may be ischemic necrosis of the hard and soft palates and recurrent infection with Staphylococcus aureus.15
• Cocaine-induced cerebral vasospasm and “vasculitis.” Cocaine has multiple effects on the CNS, causing ischemic and hemorrhagic strokes that primarily result from vasospasm, cerebral vascular thrombosis, enhanced platelet aggregation, and sporadic episodes of hypertension. Endothelin-1 may be an important mediator in cocaine-induced vasospasm.
The intracerebral syndrome most frequently associated with cocaine is reversible cerebral vasoconstriction syndrome (RCVS).16 This disorder is characterized by prolonged but reversible vasoconstriction of the cerebral arteries that usually is associated with a severe, acute onset and recurrent headaches; other neurological features may be present. Diagnostic features, as suggested by Calabrese and colleagues,16 include radiographic evidence of multifocal segmental cerebral artery vasoconstriction (conventional, CT, or MR angiography), an absence of subarachnoid hemorrhage, normal results of cerebrospinal fluid analysis, severe acute headaches, and reversibility of the angiographic abnormalities within 12 weeks of onset.
Drug-induced cerebral artery vasospasm, such as with cocaine, is a major consideration in the differential diagnosis. Management consists of empiric therapy with calcium channel blockers (nimodipine and verapamil) or other agents; short courses of corticosteroids; magnesium sulfate; or clinical observation.
Cerebral vasculitis also has been postulated to play a role in the neurovascular effects of cocaine. In 1995, Merkel and coworkers17 reviewed 8 cases of cocaine-induced cerebral vasculitis; patients’ mean age was 28 years. Time between last known cocaine use and brain biopsy varied from hours to 6 months. In 6 cases, a brain biopsy revealed small-vessel angiitis. One patient died, and autopsy showed no medium- or large-vessel vasculitis. The authors attributed this complication of cocaine use to the chemical mediators released in the context of endothelial inflammation and a cytokine cascade with resultant vasospasm and arteritis.
Two studies published in 1996 reviewed autopsy samples from cocaine users with fatal cerebral hemorrhages (14 autopsy cases in one and 10 in another). Subarachnoid, intracerebral, or parenchymal hemorrhages were seen in all the specimens, but there was no evidence of vasculitis.
• Cocaine and coronary artery aneurysms. Cocaine may have multiple deleterious effects on the cardiovascular system. Acute use may increase blood pressure and heart rate via α- and -adrenergic stimulation (increased oxygen demand) and coronary spasm via α-adrenergic stimulation (decreased oxygen delivery), resulting in ischemia, infarction, and arrhythmias. Long-term cocaine use may lead to accelerated atherosclerosis, myocarditis, contraction-band necrosis, and dilated or hypertrophic cardiomyopathy18 and coronary aneurysms.19
Conclusion
Cocaine always should be included in the differential diagnosis in patients who have appropriate epidemiological risks and a picture of RCVS or unexplained cerebral arteritis; skin lesions; oronasal/palatal destructive deformities, with no systemic features; neutropenia/granulocytosis; and a number of autoimmune laboratory abnormalities. A skin biopsy may be helpful in some patients; this biopsy may reveal vasculopathy, thrombosis, or leukocytoclastic vasculitis.
Cocaine also has been implicated anecdotally in urticarial vasculitis, Churg-Strauss vasculitis, Henoch-Schnlein purpura, lupus and lupus nephritis, Stevens-Johnson syndrome, and antiphospholipid antibody syndrome.3,20-24 Skin lesions generally resolve over 2 to 3 weeks after cocaine/levamisole is stopped, and the autoimmune laboratory abnormalities generally resolve over 2 to 12 months.25
Implications for Physicians
Cocaine may mimic myriad medical conditions, especially rheumatologic conditions. Whether cocaine itself or the levamisole contaminant causes the autoimmune response remains unclear. Management strategies include making the appropriate diagnosis, gaining knowledge of the various rheumatologic disorders that cocaine can mimic, conducting prompt testing for cocaine and levamisole contaminant, and advocating immediate cessation of cocaine use.
Laboratory testing may consist of a complete blood cell count, basic chemistries and urinalysis with toxicology, and appropriate imaging; when patients present with altered mental status or focal neurological symptoms, lumbar puncture with imaging needs to be considered. Consultation with various medical subspecialties, such as rheumatology, infectious disease, neurology, and dermatology, should be considered. Patients with neutropenia should be monitored for infections and treated appropriately.
The role of corticosteroids in these cases is controversial; they may be used for a short duration to help resolve localized inflammatory features. The use of other immunosuppressive medications, especially over the long term, is strongly discouraged. However, if there is diagnostic confusion as with cerebral vasculitis resulting from cocaine versus other causes, strong immunosuppression should be considered while the workup is under way. Thus, familiarity with the vast array of cocaine-induced conditions is critical for practicing rheumatologists so as to spare patients unnecessary and potentially toxic immunosuppressive therapies.
References:
REFERENCES
1. Substance Abuse and Mental Health Services Administration. Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: US Dept of Health and Human Services; 2009. NSDUH series H-36, HHS publication SMA 09-4434.
2. Petersen RC. History of cocaine. NIDA Res Monogr. 1977;(series 13):17-34.
3. Englander M, Davie JM. Antiphospholipid antibodies, cocaine use, and stroke in a young woman. J Clin Rheumatol. 1997;3:224-226.
4. Deng SX, Bharat N, Fischman MC, Landry DW. Covalent modification of proteins by cocaine. Proc Natl Acad Sci U S A. 2002;99:3412-3416.
5. Jankowski MM, Ignatowska-Jankowska B, Glac W, Swiergiel AH. Cocaine administration increases CD4/CD8 lymphocyte ratio in peripheral blood despite lymphopenia and elevated corticosterone. Int Immunopharmacol. 2010;10:1229-1234.
6. Casale JF, Corbeil EM, Hays PA. Identification of levamisole impurities found in illicit cocaine exhibits. Microgram J. 2008;6(3-4):82-89.
7. Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
8. Ching JA, Smith DJ Jr. Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
9. Scheinberg MA, Bezerra JB, Almeida FA, Silveira LA. Cutaneous necrotising vasculitis induced by levamisole. Br Med J. 1978;1:408.
10. Barroso M, Gallardo E, Queiroz JA. Bioanalytical methods for the determination of cocaine and metabolites in human biological samples. Bioanalysis. 2009;1:977-1000.
11. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians [published correction appears in Mayo Clin Proc. 2008;83:851]. Mayo Clin Proc. 2008;83:66-76.
12. Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24: 151-172.
13. de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after use of cocaine probably adulterated with levamisole. Dermatology. 2011;223:25-28.
14. Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum. 2004;50:2954-2965.
15. Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum. 2008;58:1546-1551.
16. Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34-44.
17. Merkel PA, Koroshetz WJ, Irizarry MC, Cudkowicz ME. Cocaine-associated cerebral vasculitis. Semin Arthritis Rheum. 1995;25:172-183.
18. Kloner RA, Rezkalla SH. Cocaine and the heart. N Engl J Med. 2003;348:487-488.
19. Satran A, Bart BA, Henry CR, et al. Increased prevalence of coronary artery aneurysms among cocaine users. Circulation. 2005;111:2424-2429.
20. Hofbauer GF, Burg G, Nestle FO. Cocaine-related Stevens-Johnson syndrome. Dermatology. 2000;201:258-260.
21. Orriols R, Muñoz X, Ferrer J, et al. Cocaine-induced Churg-Strauss vasculitis. Eur Respir J. 1996;9:175-177.
22. Chevalier X, Rostoker G, Larget-Piet B, Gherardi R. Schoenlein-Henoch purpura with necrotizing vasculitis after cocaine snorting. Clin Nephrol. 1995;43:348-349.
23. Rivera TL, Belmont HM, Weissmann G. Systemic lupus erythematosus in 6 male cocaine users at Bellevue hospital. J Rheumatol. 2009;36:2854-2855.
24. Williams EL, Endean AL, Edwards CJ. Myocardial infarction in a young man with antiphospholipid syndrome and cocaine use. Lupus. 2007;16:444-446.
25. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
26. Graf J, Lynch K, Yeh CL, et al. Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63:3998-4001.
27. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia-a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
28. Ullrich K, Koval R, Koval E, et al. Five consecutive cases of a cutaneous vasculopathy in users of levamisole-adulterated cocaine. J Clin Rheumatol. 2011;17:193-196.
29. Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
30. Khan TA, Cuchacovich R, Espinoza LR, et al. Vasculopathy, hematological, and immune abnormalities associated with levamisole-contaminated cocaine use. Semin Arthritis Rheum. 2011;41:445-454.
31. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leukopenia. Semin Arthritis Rheum. 2011;41:434-444.
32. Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2011 Nov 15; [Epub ahead of print].
33. Zwang NA, Van Wagner LB, Rose S. A case of levamisole-induced systemic vasculitis and cocaine-induced midline destructive lesion: a case report. J Clin Rheumatol. 2011;17:197-200.
34. Farmer RW, Malhotra PS, Mays MP, et al. Necrotizing peripheral vasculitis/vasculopathy following the use of cocaine laced with levamisole. J Burn Care Res. 2012;33:e6-e11.
35. John S, Manda S, Hamrock D. Cocaine-induced thrombotic vasculopathy. Am J Med Sci. 2011;342:524-526.
36. Buchanan JA, Vogel JA, Eberhardt AM. Levamisole-induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole. J Med Toxicol. 2011;7:83-84.
37. Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010;152:758-759.
38. Farhat EK, Muirhead TT, Chaffins ML, Douglass MC. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;146:1320-1321.
39. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010; 37:1212-1219.