Riociguat for Systemic Sclerosis Digital Ulcers May Require Longer Treatment
A proof of concept clinical trial for riociguat (Adempas, Bayer) for patients with systemic sclerosis indicates that longer treatment periods may be necessary to heal digital ulcers. In this trial, the treatment wasn't effective in reducing the number of digital ulcers.
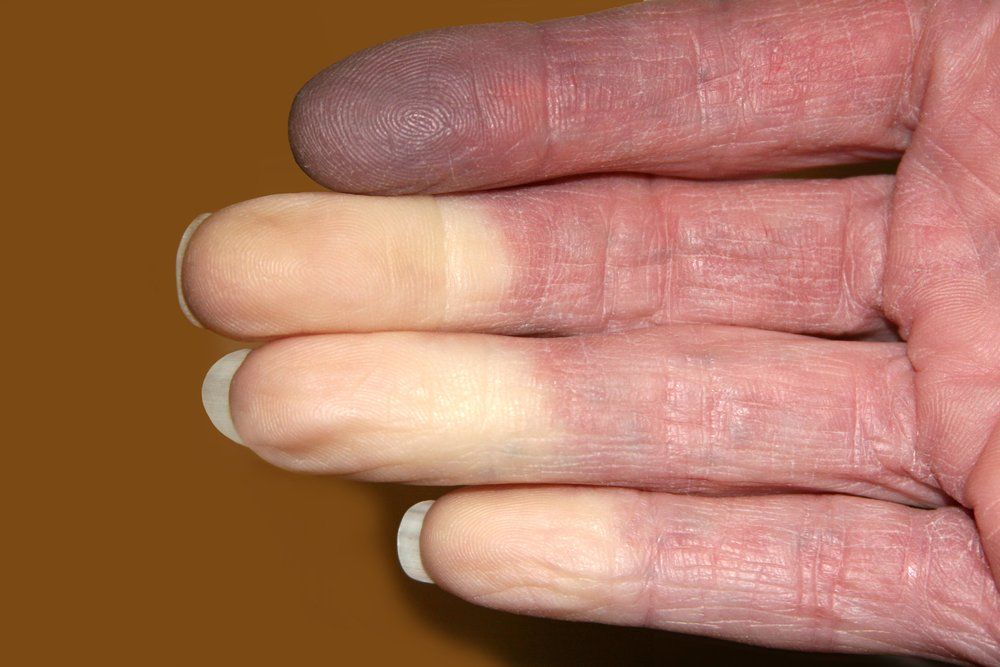
Riociguat (Adempas, Bayer) for patients with systemic sclerosis digital ulcerls may work best if taken for longer priods of time, shows a study published in Arthritis Research and Therapy. The condition is associated with Raynaud's phenomenon shown here in this image. (©TwinsChoiceShutterstock.com)
A proof of concept clinical trial for riociguat (Adempas, Bayer) for patients with systemic sclerosis indicates that longer treatment periods may be necessary to heal digital ulcers. In this trial, the treatment wasn't effective in reducing the number of digital ulcers.
The findings were published Sept. 3 in Arthritis Research and Therapy.
Digital ulcers occur in around 30 percent of patients with systemic sclerosis each year and are associated with substantial morbidity that can escalate to gangrene and amputation in around 15 percent of patients. Bosentan is approved in Europe to reduce the number of new digital ulcers in patients with systemic sclerosis, but there are no drugs approved in the U.S. for the treatment of digital ulcers in these patients.
Previous studies have reported that riociguat was well tolerated in patients with Raynaud’s phenomenon and resulted in improved digital blood flow in some cases, while the drug may also reduce Raynaud’s phenomenon symptoms and attack frequency compared with placebo in patients with early diffuse cutaneous systemic sclerosis.
Researchers assessed the efficacy and safety of 16 weeks of riociguat treatment in a randomized, placebo-controlled pilot clinical trial of 17 patients (nine treatment, eight placebo) with systemic sclerosis-associated digital ulcers. The initial trial was followed by an optional open-label extension study for an additional 16 weeks with six treatment and six placebo participants.
At baseline, the mean net ulcer burden was 2.5 (2.0) in the placebo and 2.4 (1.4) in the riociguat. No significant treatment difference was observed in the change from baseline to 16 weeks in net ulcer burden (adjusted mean treatment difference − 0.24, 95% CI (− 1.46, 0.99), p = 0.70). Statistically significant elevation of cGMP was observed at 16 weeks in the riociguat group (p = 0.05), but there were no other changes in biomarkers.
In the open-label extension phase, complete healing of digital ulcers was observed, a finding that requires confirmation in a larger trial, researchers wrote.
“We did not find any statistical or clinically meaningful differences in the net ulcer burden and other secondary outcome measures,” including patient-reported symptoms of Raynaud’s phenomenon, “but we showed target engagement, as exemplified by an increase in plasma cGMP in the riociguat group,” wrote the authors, led by Dinesh Khanna, M.D., of the University of Michigan.
The safety profile of riociguat was consistent with that observed previously in patients with pulmonary arterial hypertension, with no new safety events identified.
REFERENCE
Vivek Nagaraja, Cathie Spino, Erica Bush, et al. “A multicenter randomized, double-blind, placebo-controlled pilot study to assess the efficacy and safety of riociguat in systemic sclerosis-associated digital ulcers.”Arthritis Research and Therapy. September 3, 2019. doi: 10.1186/s13075-019-1979-7