Article
Risk of Cutaneous Squamous Cell Carcinoma Higher in Patients with AK
Author(s):
The risk of cSCC was significantly higher in patients who received treatment for actinic keratosis.
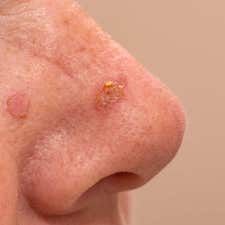
A new investigation from the Netherlands found that the risk of cutaneous squamous cell carcinoma (cSCC) was higher in patients with Olsen grade III actinic keratosis, and that this risk increased substantially in those who received additional treatment.
With a global prevalence of 11-60%, actinic keratosis has been frequently diagnosed by dermatologists and general practitioners, and the suggestion that it can eventually lead to invasive cSCC has been debated.
Additionally, there is limited evidence of the treatment of actinic keratosis decreasing the risk of invasive cSCC development.
As such, investigators led by Shima Ahmady, MD, Department of Dermatology at Maastricht University Medical Center, evaluated the risk of cSCC and other contributing factors to increased risk in patients with actinic keratoses.
Ahmady and colleagues utilized data from a 4-year follow-up period that included patients who had participated in a randomized clinical trial comparing 4 field-directed actinic keratosis treatments.
The long-term risk of invasive cSCC was evaluated in patients with actinic keratosis who were treated with either 5% fluorouracil cream, 5% imiquimod cream, methylamino levulinate PDT, or 0.015% ingenol mebutate gel.
Patients were enrolled from November 1, 2014, and March 31, 2017 from the dermatology departments of 4 hospitals in the Netherlands. The final patient population included those 18 years or older with Fitzpatrick skin types I to IV with a minimum of 5 actinic keratosis lesions within a treatment area of 25 to 100 cm2 in the head and neck region.
Following enrollment, patients were randomly assigned to 1 of the 4 treatment groups.
The primary outcomes included the proportion of patients with invasive cSCC in the target area during follow-up, with secondary outcomes being the associations between risk of invasive cSCCC and a priori defined potential prognostic factors.
A total of 624 patients were included in the study, 89.4% of whom were males with the median age being 73 years. Among these patients, 26 were diagnosed with a histologically proven invasive cSCCC in the target area during follow-up.
Investigators found that the total 4-year risk of developing cSCC in a previously treated area of actinic keratosis was 3.7% (95% CI, 2.4%-5.7%). This finding varied from 2.2% (95% CI, 0.7%-6.6%) in patients treated with fluorouracil to 5.8% (95% CI, 2.9%-11.3%) in patients treated with imiquimod.
Additionally, Ahmady and colleagues found that patients with severe actinic keratosis had an increased risk of 20.9% (95% CI, 10.8%-38.1%) which was higher still in patients who required additional treatment (33.5%; 95% CI, 18.2%-56.3%).
The team noted that there are no studies that have found imiquimod treatment to lead to an increased risk of developing cSCC. However, the use of ingenol mebutate had recently been suspended by the European Medicines Agency after reportedly being linked to increased risk of developing skin cancer.
“In this secondary analysis of a randomized clinical trial, the risk of invasive cSCC was highest in patients with Olsen grade III AK lesions and was substantially increased in patients who received additional treatment,” the team wrote. “We therefore recommend close follow-up of these patients.”
The study, "Risk of Invasive Cutaneous Squamous Cell Carcinoma After Different Treatments for Actinic Keratosis," was published online in JAMA Dermatology.





