Six New Insights About RA Treament from the AMPLE Trial
EULAR 2013: Two-year data from a head-to-head comparison of abatacept and adalimumab show them comparably effective for rheumatoid arthritis. But the former is less costly with fewer noteworthy adverse effects.
Two-year results from the first head-to-head trial of the biologics abatacept (Orencia) and adalimumab (Humira) show them nearly indistinguishable in terms of efficacy for rheumatoid arthritis (RA), when added to background methotrexate. However, abatacept is somewhat less costly and less likely to be discontinued due to adverse events.
The two biologics are far preferable to other options for patients who don't respond to methotrexate, judging from other studies also reported at the European League Against Rheumatism (EULAR) conference in Madrid using data from the same trial, the international multicenter AMPLE study. But this secondary conclusion assumes that you have identified rheumatoid arthritis early, begun treatment promptly, and are able to achieve remission quickly.
Researchers in AMPLE randomized 646 biologic-nave patients, who had moderate to high RA and had not responded to at least 15 mg/wk of methotrexate, to take either 125 mg of subcutaneous abatacept (ABA) weekly or 40 mg subcutaneous adalimumab (ADA) bi-weekly, plus a stable continuing dose of methotrexate. (The two drugs dampen immunity by different means of interfering with T-cell function. ABA blocks two receptors on B cells to inhibit their stimulation of T cells, while ADA inhibits T-cell activation by blocking two receptors on the cytokine tumor necrosis factor alpha.)
Fundamentally, the two-year results echo one-year data from AMPLE, published last January in Arthritis & Rheumatism. Speakers at EULAR also offered new insights from the first year's information.
• Judging from core two-year outcome data from AMPLE, ADA and ABA are similar in terms of efficacy, but adverse events differ somewhat. By day 729 of the trial, 79.2% (252/318) of patients on ABA and 74.7% (245/328) of those on ADA were still enrolled and under treatment. Responses for American College of Rheumatology outcomes measures ACR 20, 50, 70, and 90 were similar, as were those for the HAQ health-quality assessment scale.
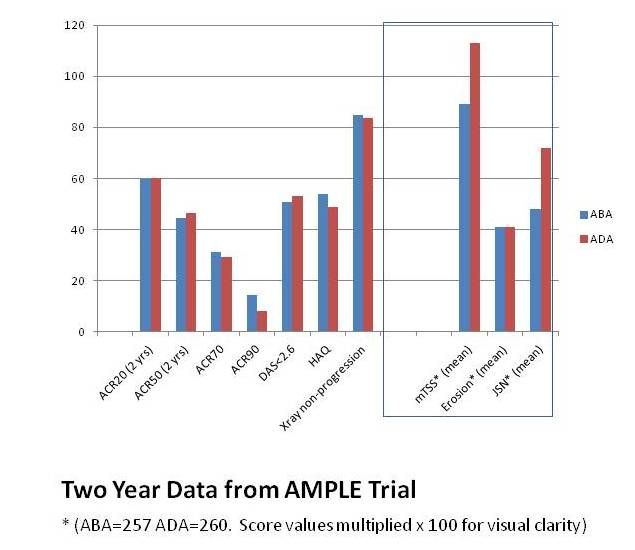
Patients on ABA experienced more autoimmune adverse effects (AEs), but fewer infections, including opportunistic infections, and fewer discontinued treatment due to AEs.
• Reaching remission costs more than $60,000, and staying there costs roughly $350 a day, according to cost-efficacy comparisons carried out on data from AMPLE. Evidently ABA achieves the same results for roughly 10% less expense The researchers analyzed cost per patient over one year, based on approved net costs in US dollars or in euros in Germany, using a reduction in of >0.3 units in the HAQ-DI score or improvements in activity limitation (time over the previous 30 days when the patient was limited in performing work or non-work activities) as measures of effectiveness. The cost of achieving remission is $63,232 for ABA and $67,947 (7% more) for ADA. Every day without activity limitation costs $323 for a patient taking ABA and $380 per day on ADA (15% more).
• ADA and ABA are equally effective in improving quality of life in RA, although more patients improve quickly with abatacept. Patient-reported outcomes for work productivity, physical activity, and independence from year one of AMPLE were identical after 365 days, although patients taking ABA had shown greater improvements in work and physical activity at about the half-year point (169 days). Scores for independence, both psychosocial and physical, were nearly identical.
• One-year data on physical function and radiographic outcomes from AMPLE show that patients who achieve early remission continue well throughout the year. Across all definitions of remission or disease activity (DAS28, RAPID3, CDAI, and SDAI), more than 60% of patients who achieved remission at day 85 and day 169 showed good HAQ (quality of life) scores by the end of the first year. More than 80% of patients who achieved remission or low disease activity at day 85 and day 169 were radiographic non-progressors (with a change in mTSS < 2.8) at day 365. These improvements in physical function and radiographic outcomes were “consistent” between the 2 groups.
• An IV loading dose probably isn't necessary for patients taking subcutaneous abatacept, according to analysis of data from AMPLE and from the multicenter ACQUIRE study. Patients participating in ACQUIRE (results published in 2011 in Arthritis and Rheumatism) took the same weekly dose of ABA used in the AMPLE trial, but began with a 10 mg/kg IV loading dose. The analysis
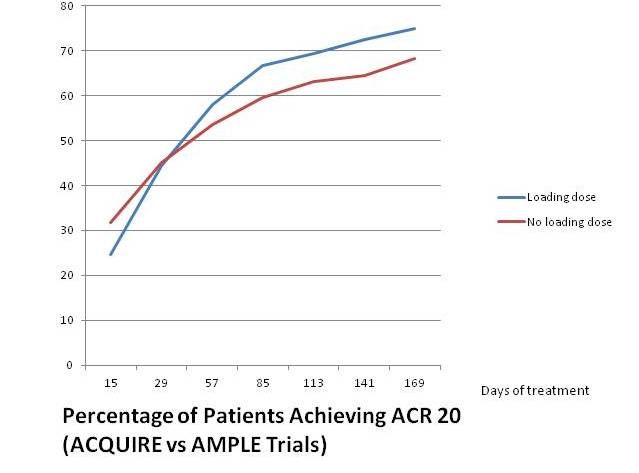
reported at EULAR this year compared data for 318 patients from the intent-to-treat population in AMPLE with those for 736 patients in ACQUIRE. The mean initial disease duration at baseline was far different (1.8 years for AMPLE versus 7.6 years for ACQUIRE), but initial disease states were similar (DAS28 6.2 for AMPLE versus 5.5 for ACQUIRE, and HAQ-DI 1.72 for AMPLE versus 1.5 for ACQUIRE.) Efficacy results for subcutaneous treatment were similar with and without the loading dose (see chart at left).
• RA patients caught early now can expect much better results with today's biologics than those who began TNF-alpha inhibitor treatment later in the disease. This gratifying but perhaps predictable result comes from a post-hoc cross-trial comparison of AMPLE data and results from the ATTEST study, which matched IV ABA against infliximab.
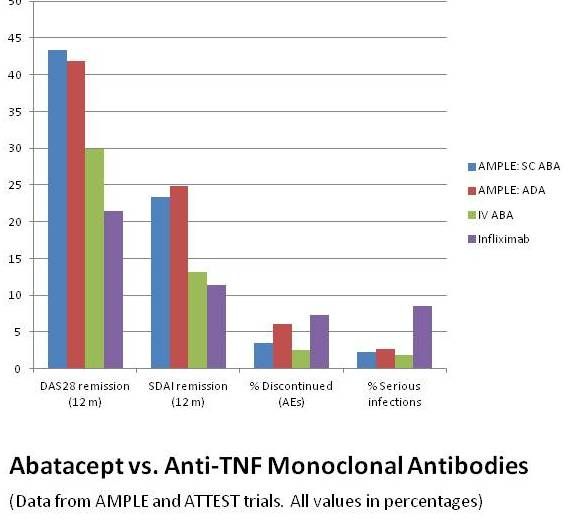
Patients in both ATTEST and AMPLE had active RA and were inadequate responders to methotrexate, but those in the earlier ATTEST trial were farther along, with disease durations about 7 years for both treatment arms (as against less than 2 years for both treatment arms in AMPLE), when they began intravenous treatment with either ABA or infliximab.
Note these conclusions from the data shown at right: Far more AMPLE patients achieved remission after one year. Patients taking infliximab had more discontinuations due to AEs, as did those on adalimumab in the AMPLE trial, and those receiving infliximab had more serious infections.