Article
Sublingual Immunotherapy Improves Severity of Atopic Dermatitis
Author(s):
New sensitizations to 2 or more allergens between baseline and 12 months were significantly lower in the sublingual group, compared to the control group.
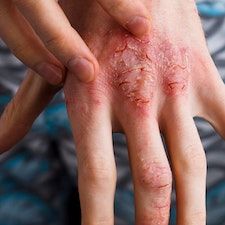
Sublingual immunotherapy is showing efficacy in treating patients with atopic dermatitis who are allergic to house dust mites.
A team, led by Minju Kim, Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, evaluated the efficacy of sublingual immunotherapy for pediatric patients aged 5-17 years with atopic dermatitis who were allergic to house dust mites.
The Trial
In the open-label, controlled, randomized trial, the investigators examined 60 patients from June 2015 and February 2018 at a specialist allergy center in South Korea. The patients were split in half between a group that received sublingual immunotherapy for 12 months and a control group.
The investigators evaluated the participants using specialist scores and specific immunoglobulin and skin prick tests.
Results
The results show sublingual immunotherapy significantly decreased the mean Scoring Atopic Dermatitis measurements in the sublingual group from baseline (30.2±10.7) to 3 months (20.7±8.5). The effects were still visible at 12 months (21.5±12.4).
The investigators also found that the levels of Dermatophagoides farina-specific immunoglobulin G4 were significantly higher in the treatment group from baseline (0.6±0.5) to 12 months (1.0±0.7), compared to the control group that saw no significant changes.
New sensitizations to 2 or more allergens between baseline and 12 months were significantly lower in the sublingual group (21.4%), compared to the control group (54.2%).
“Sublingual immunotherapy improved disease severity and prevented new sensitizations in children with atopic dermatitis who were allergic to dust mites,” the authors wrote.
JAK Inhibitors
Earlier this year, investigators found janus kinase (JAK) inhibitors appear to be effective treatments for patients with atopic dermatitis, according to a systematic review of 14 randomized controlled trials.
The trials assessed 3 different oral JAK inhibitors, abrocitinib (Cibinqo), baricitinib (Olumiant), and upadacitinib (Rinvoq).
Non-responders to topical therapy are sometimes treated with phototherapy, systemic therapies like methotrexate and cyclosporine, or other immunosuppressive agents.
Altogether, the authors found 14 trials, including 7,051 total subjects. The trials assessed abrocitinib (100 and 200 mg), baricitinib (1, 2, and 4 mg), or upadacitinib (15 and 30 mg) for the treatment of moderate-to-severe AD.
All 3 therapies led to improvement in all 4 outcome metrics compared to placebo, the authors found. However, one therapy stood out. The investigators said upadacitinib at 30 mg led to the best results in all 4 outcomes among all of the drugs and dosages.
In terms of safety, the authors said their meta-analysis showed patients taking JAK inhibitors had a higher percentage of herpes zoster infection than the placebo-controlled group. In addition, they noted that the immunomodulatory properties of JAK inhibitors have raised concerns about an increased risk of malignancy.
The team added that 8 patients taking upadacitinib and 2 patients receiving placebo were reported to have malignancies. However, most of the cancers were not believed to be related to the drug and suggested longer-term safety data would be required to fully understand the risk of malignancy for patients taking these drugs.
The study, “Sublingual immunotherapy may be effective in reducing house dust mite allergies in children with atopic dermatitis,” was published online in Acta Paediatrica.





