Article
Allergan Issues Voluntary Recall of Taytulla Contraceptive
Author(s):
Specifically, the first 4 days of the capsules were packaged with 4 non-hormonal placebo capsules in place of their active counterparts.
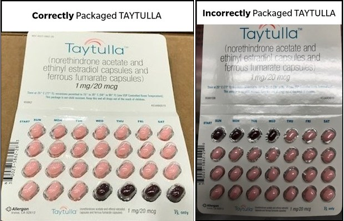
Comparison of the packaged Taytulla products
Credit: Allergan plc
Allergan has issued a voluntary recall in the United States for a single lot of its norethindrone acetate and ethinyl estradiol capsules and ferrous fumarate capsules (Taytulla) physician sample packs, due to the incorrect placement of placebo pills in the pack.
Caught through a physician report of the pregnancy prevention product, the lot in question has been identified by Allergan as Lot #5620706 (Expiry May 2019). The packs were of the 1 mg/20 mcg 6x28 quantity.
Specifically, the first 4 days of the capsules were packaged with 4 non-hormonal placebo capsules in place of their active counterparts. The product was distributed nationally to health care providers, according to Allergan.
The recall is being conducted with the knowledge of the US Food and Drug Administration (FDA).
The pack itself has 24 active softgel capsules—designated by their pink color—with a “WC” on the outer shell, intended to be administered for 24 consecutive days, and 4 softgel placebo capsules also stamped with a “WC”—identifiable by their maroon color—meant to be taken for the final 4 days of the 28-day period.
If taken out of order, the oral contraceptive can place the individual user at risk for failure in contraception and result in unintended pregnancy. This unintended reversal of order, Allergan has stated, “may not be apparent to either new users or previous users of the product, increasing the likelihood of taking the capsules out of order. If patients have concerns regarding the possibility of an unintended pregnancy they should consult their physician.”
Allergan has begun notified customers with a recall letter and is organizing itself for the return of all the recalled sample packs from the Lot #5620706. Those who are in possession of the sample pack product with that associated lot number are being asked to notify their physician to set up the return.
Additionally, if a patient is concerned and received a pack since August 27, 2017, it is recommended that they contact their physician. Allergan has stated that those with questions regarding the recall can contact the company by phone at 800-678-1605 from 8 AM to 8 PM EST, Monday through Friday.
Adverse events experienced with the use of this product can be reported to the FDA's MedWatch Adverse Event Reporting Program either online, by regular mail or by fax. To complete and submit the report online, visit: www.fda.gov/medwatch/report.htm. To do so via regular mail or by fax, the downloadable form can be found here: www.fda.gov/MedWatch/getforms.htm or customers can call 1-800-FDA-1088 to request it. It must then be completed, and return addressed on the pre-addressed form, or submitted by fax to 1-800-FDA-0178.





