Combining Lyrica and Savella Reduces Fibromyalgia Pain More Effectively Than Monotherapy
Fibromyalgia patients who don't respond to Lyrica (pregabalin) may see their pain scores significantly improve after adding Savella (milnacipran) to their treatment regimens.
Fibromyalgia patients who don’t respond to Pfizer Inc.'s Lyrica (pregabalin) may see their pain scores significantly improve after adding Savella (milnacipran) to their treatment regimens, according to research published in the June 2013 edition of Therapeutic Advances in Musculoskeletal Disease.
For their open-label study supported by funding from Savella manufacturer Forest Laboratories Inc., Philip J. Mease, MD, from the University of Washington School of Medicine, and full-time employees of the drugmaker’s research arm randomized a total of 364 fibromyalgia patients who were classified as incomplete responders after a four- to 12-week pregabalin treatment period to receive 300 milligrams or 450 milligrams of once-daily pregabalin alone (n=180) or with 100 milligrams of once-daily milnacipran (n=184).
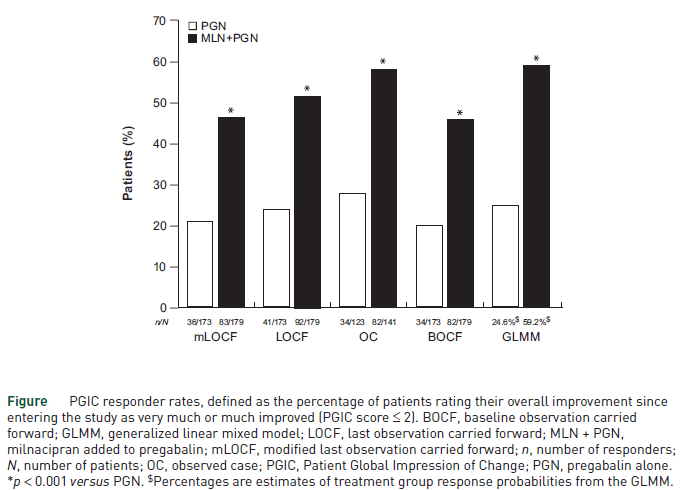
After 11 weeks, the proportion of patients responding to treatment was “significantly higher with milnacipran added to pregabalin (46.4 percent) than with pregabalin alone (20.8 percent; p < 0.001),” and “the percentage of patients with at least 30 percent pain improvement was approximately twice as high with milnacipran plus pregabalin (45.8 percent) than with pregabalin alone (19.7 percent),” the authors wrote. (Figure)
Examining the safety and tolerability of combining the two therapies — which are separately approved by the US Food and Drug Administration for the treatment of fibromyalgia — the authors found that adding Savella to Lyrica didn’t exacerbate the previously reported treatment-related adverse effects associated with milnacipran or pregabalin monotherapy, including nausea, headache, constipation, insomnia, dizziness, and fatigue. In fact, the “incidence of some side effects in patients receiving milnacipran plus pregabalin was lower than previously found with milnacipran monotherapy,” the authors added.
“Individual patient responses to monotherapies, as well as to combination therapies of medications with different mechanisms of action, highlight the ongoing need to study potential biomarkers of fibromyalgia to improve diagnosis and develop more targeted treatment strategies,” the researchers concluded. “For some patients requiring more than one fibromyalgia medication, the addition of minacipran to pregabalin may be a favorable treatment option.”