Article
FDA Approves Hepatitis C Drug Effective Without Added Interferon
Sofosbuvir, newly approved for chronic hepatitis C infection, is the first possible therapy for some infected patients with rheumatic diseases. Treatment costs $1000 a day.
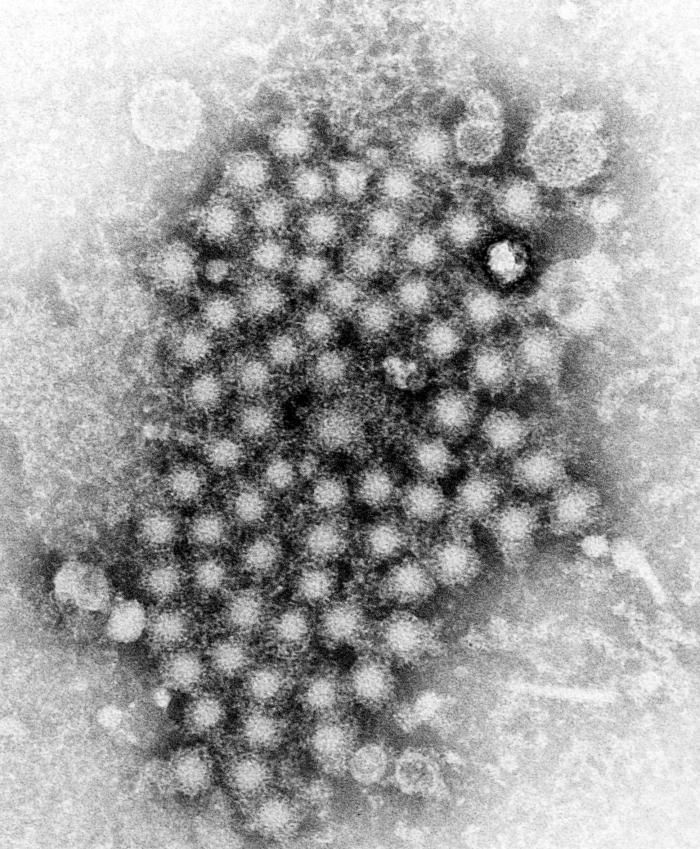
The US Food and Drug Administration has approved Sovaldi (sofosbuvir), which has demonstrated safety and efficacy for some types of chronic hepatitis C virus (HCV) infection without concomitant interferon. This is the first drug that would help patients affected with chronic HCV whose rheumatic conditions prevent them from taking interferon.
This represents a "significant shift in the treatment paradigm for some patients with chronic hepatitis C,” said Edward Cox, M.D., director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Sovaldi is the second drug approved by the FDA in the past two weeks to treat chronic HCV infection, after simeprevir (Olysio) approved on November 22. It is the third to be approved with the "breakthrough therapy" designation, which allows accelerated approval for drugs that demonstrate a substantial improvement over available therapies for patients with serious or life-threatening diseases..
Sovaldi is a nucleotide analog inhibitor that blocks a polymerase essential for replication of HCV, intended to be used as a component of combination therapy with ribavirin and/or peginterferon-alfa. The approval was based on six clinical trials consisting of 1,947 treatment-naive participants coinfected with HIV and HCV, with an endpoint of continued disappearance of viral signal in the blood at least 12 weeks after the end of therapy. The drug demonstrated efficacy, among other groups, among patients who could not tolerate or take and interferon-based regimen.
The most common side effects reported in clinical study participants treated with Sovaldi and ribavirin were fatigue and headache.
Press reports have noted that according to Gilead the 12-week one-pill-a-day regimen will cost $84,000, which comes to $1,000 a day.




