FDA Approves Painless Blood Collector
New device draws blood painlessly from capillaries.
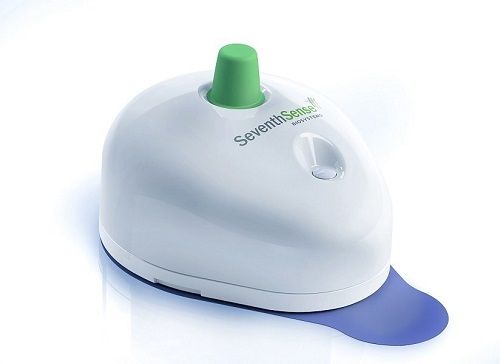
The US Food and Drug Administration (FDA) approved a device its manufacturer says can draw blood without involving the needles used in finger sticks or syringes.
Seventh Sense Biosystems of Medford, Massachusetts announced it has gotten FDA approval for a device (photo) that uses tiny needles the size of a human eyelash to painlessly get blood from capillaries.
Collection takes 2-3 minutes. The device is placed on the patient's arm and is activated by a pushbutton.
The FDA clearance is for health care personnel to collect blood to monitor hemoglobin A1c, but the company is working with the FDA to get approval for expanded use, including allowing patients to do it themselves. That could be particularly useful for patients with diabetes.
"There is a strong market need for this type of collection technology across numerous channels," the company said in a news release.
Future uses could include labs, community settings, hospitals, retail pharmacies, athlete monitoring, and clinical trials.
The company was co-founded by a Massachusetts Institute of Technology professor, Robert Langer.
"It's shocking that with all of the healthcare innovation we've seen in the past few decades blood collection has lagged so far behind and is still a primitive and difficult process for so many people, Langer said.
Known as TAP, the device will launch in the next few months, the company said.