FDA Expands Vibativ Approval to Combat Hospital-Acquired Pneumonia
Citing a need for new therapies to remedy serious diseases acquired in hospitals, the US Food and Drug Administration has expanded approval of Vibativ (telavancin) to treat bacterial pneumonia when alternative drugs aren't appropriate.
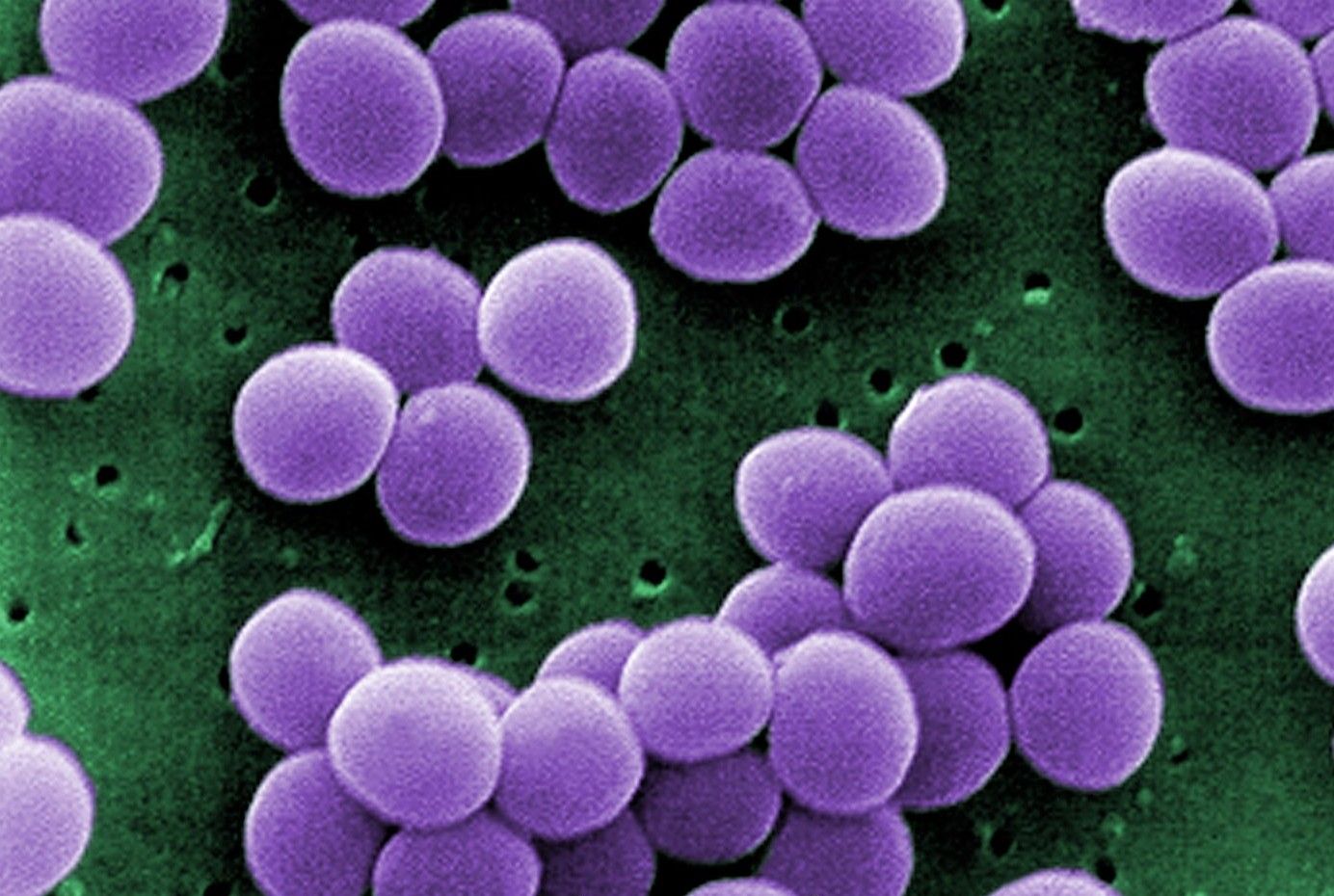
Citing a need for new therapies to remedy serious diseases acquired in hospitals, the US Food and Drug Administration has expanded approval of Vibativ (telavancin) to treat bacterial pneumonia when alternative drugs aren’t appropriate.
Originally approved by the FDA in 2009 to treat complicated skin and skin structure infections, once-daily injectable Vibativ — which is marketed by San Francisco-based Theravance Inc. — was granted an additional FDA-approved indication “for the treatment of adult patients with hospital-acquired and ventilator-associated (HABP/VABP) bacterial pneumonia caused by susceptible isolates of Staphylococcus aureus” (Staph. aureus), including methicillin-resistant (MRSA) strains, the agency said in a press release.
Although pneumonia can be traced to a wide variety of bacteria, the FDA has limited Vibativ’s expanded approval to the treatment of Staph. aureus. However, the agency noted that the type of lung infection caused by Staph. aureus — which is known as nosocomial pneumonia — is serious “because patients in the hospital and especially those on ventilators are often already very sick and usually cannot fight the infection.”
“Pneumonia is associated with one of the highest mortality rates among hospital-acquired infections and increases hospital stay and costs of care. MRSA pneumonia, in particular, is an increasingly challenging infection, as there are few approved treatments available today and resistance to current antibiotics remains a problem,” Ralph Corey, MD, a professor of medicine at the Duke University Medical Center and principal investigator for the clinical studies that supported Vibativ’s expanded FDA approval, said in a Theravance press release. “Vibativ will be a welcome addition for physicians treating hospital-acquired bacterial pneumonia … (as it) offers effectiveness in these difficult-to-treat infections when alternative therapies are not suitable.”
Two ATTAIN phase 3 clinical trials — in which 1,532 patients were randomized to receive intravenous telavancin once a day or intravenous vancomycin every 12 hours — showed comparable mortality rates between the two Staph. aureus treatments. However, in its expanded approval announcement, the FDA noted that “more patients with pre-existing kidney problems treated with Vibativ died compared to those treated with vancomycin” in the studies, so information on the lipoglycopeptide antibiotic’s potential to cause new or worsening kidney issues in patients has been added to the drug’s boxed warning.
Among all participants assigned to Vibativ in the ATTAIN trials, diarrhea was the most commonly identified side effect, the FDA said.
Theravance CEO Rick E. Winningham said the company plans to commercialize Vibativ in the US through wholesalers in the third quarter of 2013.