Innovative Tool to Determine Function in Patients With Fibromyalgia Syndrome
“Considering the fact that people with one and the same clinical condition can vary substantially in terms of disability, there is a need to expand the assessment tools,” investigators stated.
The World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) has proven to be a reliable instrument to assess function in patients with fibromyalgia syndrome (FMS), according to a study published in Advances in Rheumatology.1 It provides individual-centered care via information collected from a multidimensional perspective.
“Considering the fact that people with one and the same clinical condition can vary substantially in terms of disability, there is a need to expand the assessment tools,” investigators stated. “The instruments commonly used to assess outcomes in FMS are not aligned with all categories of functioning as defined by the International Classification of Functioning, Disability and Health(ICF). The WHODAS 2.0 is a tool that measure functioning based on the theoretical conceptual framework of the ICF.”
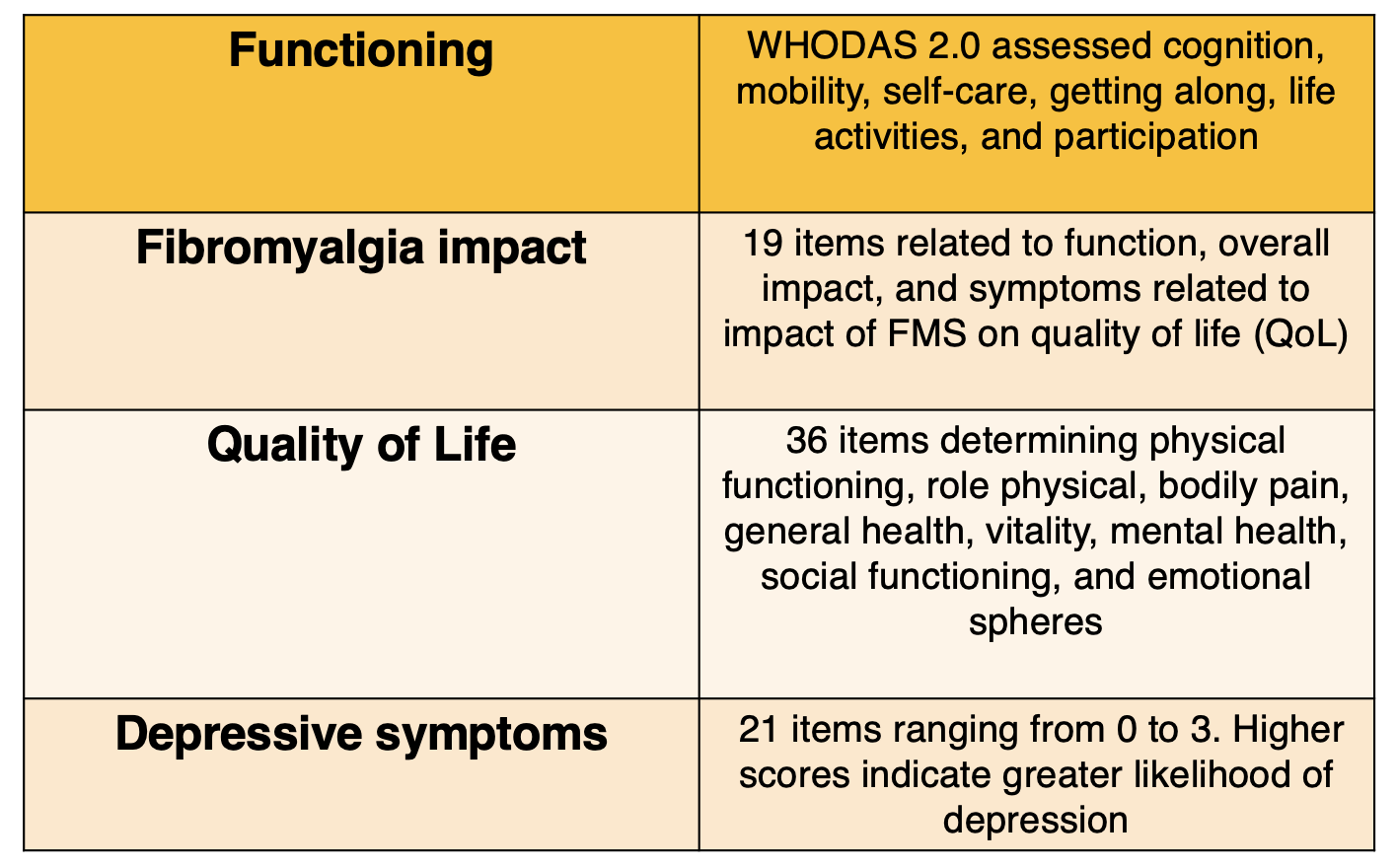
The Brazilian version of the WHODAS 2.0 tests functioning of patients with FMS using a score from 0 to 100, with higher values indicating worse functioning. Participants were also given the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), the Fibromyalgia Impact Questionnaire (FIQ), and the Beck Depression Inventory (BDI) in order to construct validity analysis. Eligible patients were aged 18 years or older and met diagnostic criteria based on the 1990 American College of Rheumatology (ACR).
Outcomes were as follows:
Data was collected between August 2017 and July 2019 and included demographics, such as gender, marital status, profession, comorbidities, and location of pain. Age, symptom evolution, and numerical rating scale (NRS) were analyzed via a questionnaire. The NRS is set on a scale of 0 to 10, with higher numbers correlating to higher levels of pain. After the initial assessment, eligible participants had a follow-up evaluation 7 days later to reapply the WHODAS 2.0 instrument.
Of the 110 participants approved by the Research Ethics Committee, most were female (97.27%) with a mean age of 44.66 years and a pain intensity level of 6.38. A total of 19.09% were unemployed due to FMS-related health issues.
The WHODAS 2.0 had a moderate to significant correlation when compared with the SF-36, BDI, and FIQ regarding the construct validity analysis. The instrument had a moderate to significant correlation to the SF-36 in all domains except for work activity. The FIQ, which focused on mobility domain and domestic activity, showed a moderate and significant correlation with the WHODAS 2.0 and cognition and getting along, based on the BDI value, showed moderate to significant correlation as well.
The test-retest reliability analysis (n = 50) had moderate to high intraclass correlation (ICC) values, except in the category regarding life activities.
A concurrent instrument was not reapplied during the second administration of the WHODAS 2.0, which limited perception of participants on the retest day. Additionally, the work activities in the functioning in life section did not apply to all patients with FMS.
“The [WHODAS 2.0] provides reliable information on individuals' health based on structures and functions of the body, activities and participation, in addition to contextual factors, that allows for individual-centered care,” investigators concluded. “Based on our findings we recommend using the instrument to assess health status and to monitor health interventions.”
Reference:
Barreto MCA, Moraleida FRJ, Graminha CV, Leite CF, Castro SS, Nunes ACL. Functioning in the fibromyalgia syndrome: validity and reliability of the WHODAS 2.0. Adv Rheumatol. 2021;61(1):58. Published 2021 Sep 16. doi:10.1186/s42358-021-00216-1