Intervention Method Aims to Reduce Fatigue in Patients with RA
The feasibility study, “Fatigue – Reducing if Effects through individualized support Episodes in Inflammatory Arthritis” (FREE-IA), tested the practicality of training, intervention delivery, and outcome collection prior to a trial that would analyze clinical and cost-effectiveness.
A manualized, cognitive-behavioral intervention designed to reduce fatigue in patients with rheumatoid arthritis (RA) was shown to be effective in reducing impact and severity of fatigue, training rheumatology health professionals (RHPs), and exhibited a high retention rate in both recruitment and follow-up of patients. Although there was no control group, improvements suggest the potential promise of an intervention and support a definitive trial to test effectiveness, according to a study published in BMJ Open.1
“Although the clinical manifestations vary, fatigue is a prevalent and often disabling symptom across types of IA6–8 It is experienced by patients as a fluctuating, unpredictable symptom that impacts on all aspects of daily life,” investigators noted. “An international study of >6000 IA patients found that 1 out of every 2 was severely fatigued, defined as scoring ≤35 on the Short-Form (SF-36) Health Survey Vitality Scale. Despite the high prevalence and impact of the symptom, patients perceive that often their fatigue is not addressed in rheumatology consultations.”
The single-arm feasibility study, titled “Fatigue – Reducing if Effects through individualized support Episodes in Inflammatory Arthritis” (FREE-IA), tested the practicality of RHP training, intervention delivery, and outcome collection prior to a trial that would analyze clinical and cost-effectiveness. Eligible patients were adults with a confirmed inflammatory arthritis diagnosis who scored ≥6/10 on the Bristol Rheumatoid Arthritis Fatigue (BRAF) Numerical Rating Scale (NRS) Fatigue Effect. After completing the training, RHPs administered 2 to 4 sessions to participants. Data was collected at baseline (T0), 6 weeks (T1), and 6 months (T2). Primary outcomes included factors relating to fatigue severity and coping, disease impact and disability, and self-efficacy and confidence to manage health.
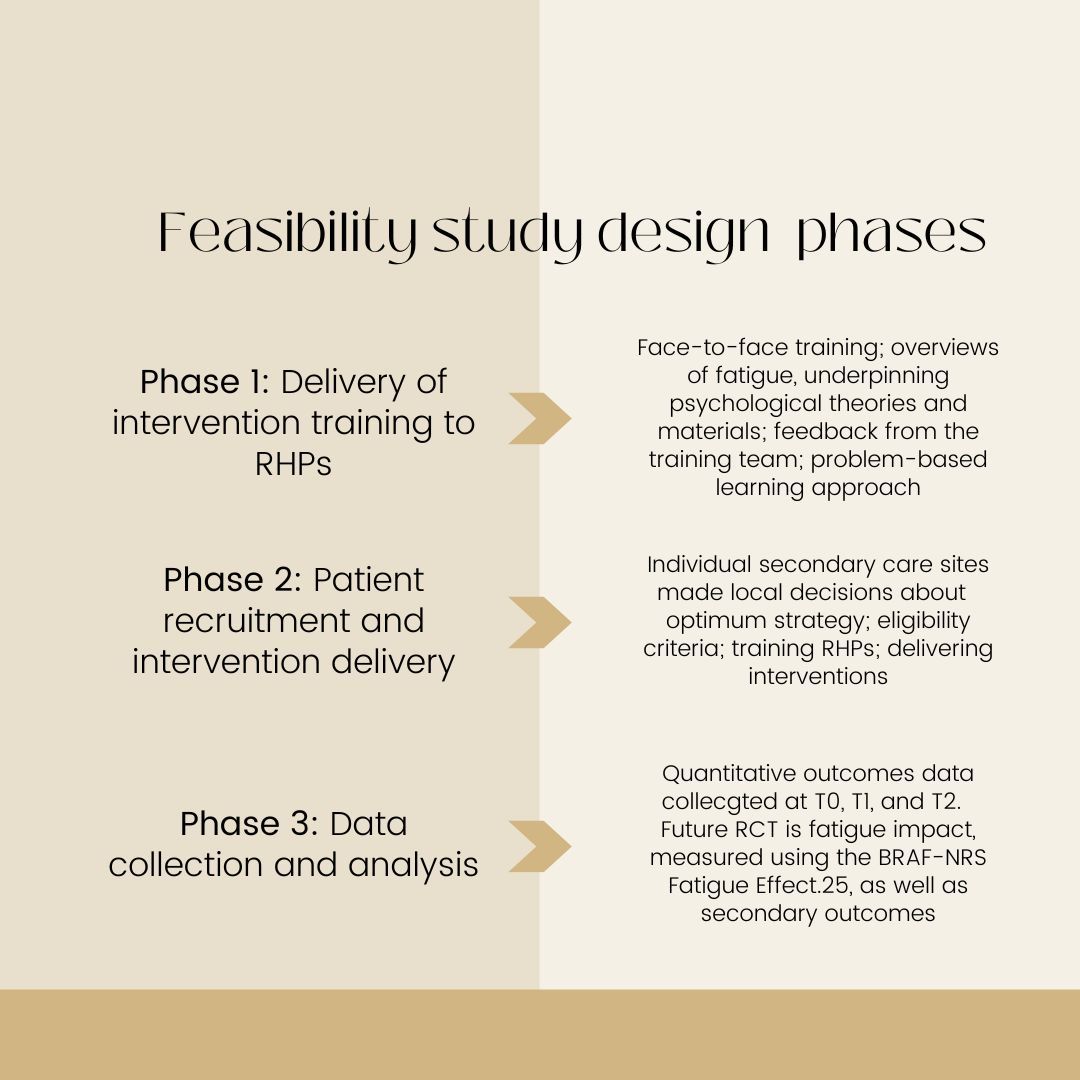
Ultimately, 8 clinicians at 5 hospitals delivered 113 sessions to 46 participants. Of the 138 primary and secondary outcomes evaluated at T1 and T2, 9.4% (n= 13) and 19.6% (n= 27) missed the response goal, respectively. Improvements were reported in all facets except for disability at either T1, T2, or both. The key cost driver for this group was medication use, with an emphasis on the cost of biologicals.
Strengths of the study included the low levels of attrition and high levels of completed outcomes coupled with the flexible, practical approach to local variation designed to further understand how the intervention could be potentially delivered in a clinical practice setting. Patient Advisory Group of the Rheumatology Department at the Bristol Royal Infirmary feedback also improved the study.
The lack of a control arm limited the study. As this was a feasibility study, data regarding health-related outcomes cannot be overinterpreted. However, results suggest that intervention may be helpful to improve patients’ fatigue. Future trials may benefit from including other outcomes, such as work productivity.
“We were able to design and deliver intervention training to RHPs, who were then able to deliver intervention sessions to participants, guided by the intervention manual,” investigators concluded. “However, it was not always possible to deliver core session 2 within the desired 2-week timeframe. Overall, our results suggest that a definitive RCT is feasible. While being cautious, outcomes were in a direction to suggest improvement in participants’ fatigue impact after attending relatively low-cost intervention sessions.”
Reference:
Dures E, Bridgewater S, Abbott B, et al. Brief intervention to reduce fatigue impact in patients with inflammatory arthritis: design and outcomes of a single-arm feasibility study. BMJ Open. 2022;12(7):e054627. Published 2022 Jul 18. doi:10.1136/bmjopen-2021-054627