Methotrexate More Effective In Psoriasis Patients Without Psoriatic Arthritis
The immunosuppressive drug was more effective and resulted in fewer adverse events in patients with psoriasis but without psoriatic arthritis.
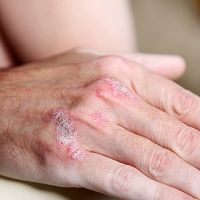
A study of methotrexate in patients with psoriasis found that the immunosuppressive drug was more effective in patients without psoriatic arthritis than those with the added condition. Additionally, patients without psoriatic arthritis had fewer adverse effects than those with psoriatic arthritis.
“Our study demonstrated that methotrexate was well tolerated by Chinese outpatients with psoriasis,” wrote study authors Kexiang Yan, MD, PhD, Yuanjing Zhang, MD, Ling Han, MD, PhD, et al. “Methotrexate appeared to be more effective and had fewer adverse effects in patients without psoriatic arthritis compared with those with psoriatic arthritis.”
This prospective, single-arm study was performed at the Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China, and enrolled 248 patients with moderate to severe psoriasis with or without psoriatic arthritis. Of those, 235 patients completed the 12-week study—none withdrew due to adverse effects.
The severity and extent of psoriasis in study participants was graded by 2 dermatologists using the Psoriasis Area Severity Index (PASI) and body surface area (BSA) scores. The mean (SD) baseline PASI score was 13.9 (7.4) and the mean (SD) baseline BSA score was 29.7 (20.8).
At week 8, the 90% reduction from baseline PASI score (PASI90) response rates were significantly higher in patients without than those with psoriatic arthritis (12 [1.9%] vs 4 [3.1%]; P = .02). This continued at week 12 (27 [25.2%] vs 19 [14.8%]; P = .049).
The PASI50 and PASI75 response rates at both week 8 and week 12 were not statistically different between the 2 groups of participants.
“Statistical differences in PASI90 response at weeks 8 and 12 were observed between patients with and without psoriatic arthritis, which can be better explained by the fact that psoriatic arthritis occurs later in psoriasis and a long-standing disease may be harder to control,” wrote the study authors.
In total, 105 participants (44.7%) experienced 1 or more adverse events, while patients with psoriatic arthritis were more likely to experience an adverse event (67 [52.3%]) than patients without psoriatic arthritis (38 [35.5]) (P = .01).
The most common adverse events were of the gastrointestinal tract (45 [19.1%]), including nausea and vomiting (20 [8.5%]), dyspepsia (20 [8.5%]), abdominal pain (5 [2.1%]), diarrhea (8 [3.4%]), and oral ulcer (3 [1.3%]). Other reported adverse effects included fatigue (29 [12.3%]), dizziness (13 [5.6%]), headache (3 [1.3%]), and hair loss (3 [1.3%]).
More patients with psoriatic arthritis experienced dizziness (12 [9.4%]) or nausea and vomiting (15 [11.7%]) than those without psoriatic arthritis (1 [0.04%], P = .007; 5 [4.6%], P = .06; respectively). Study authors noted that the difference in these 2 adverse effects might be due to genetic factors.
The study also found that methotrexate-associated abnormal hepatic function was more severe in patients with psoriatic arthritis than those without. However, the authors noted that the mechanism of hepatotoxicity continues to be unclear.
In light of those results, the investigators concluded that, “methotrexate can be recommended as first-line treatment for psoriasis without arthritis,” though they noted that larger, multicenter trials are needed to confirm this finding.
The study, “Safety and Efficacy of Methotrexate for Chinese Adults With Psoriasis With and Without Psoriatic Arthritis,” was published in JAMA Dermatology.