New Vaccine Combo for Kids Approved
The FDA approved a new vaccine combination that could mean one shot fewer for children getting their recommended routine vaccinations.
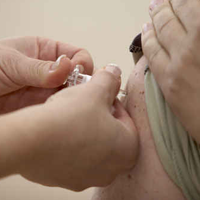
The US Food and Drug Administration (FDA) has approved a new vaccine combination to immunize children ages 4 through 6 against diphtheria, tetanus, pertussis and poliomyelitis.The new DTaP-IPV vaccine (Quadracel/Sanofi Pasteur) potentially reduces the number of routine vaccinations children will be recommended to receive, the company said in announcing the approval.
Sanofi said a getting a single dose of Quadracel has been approved as a fifth dose in the DTaP series and as a fourth or fifth dose in inactivated poliovirus vaccination series in children who have received 4 doses of the company’s Pentacel vaccine, and its DAPTACEL vaccine for diphtheria, or have received both.
Quadrecel’s approval was based on the results of a phase 3 study, which demonstrated that Quadracel has a similar safety and immunogenicity profile as the DTaP vaccine and IPV when administered separately.
The vaccine carries a small risk of allergic reactions, the company said, and may not protect all children receiving the vaccine.
Side effects possible include pain, redness, swelling at the injection site, muscle pain, fatigue and headaches.