Article
Osteoarthritis treatment update: Are NSAIDs still in the picture?
Although NSAIDs are the most effective treatment for persons who experience the pain of osteoarthritis (OA), the risk of adverse effects with NSAIDs may outweigh the benefits in many older patients. The GI risk is well known; increased attention has been paid recently to the cardiovascular risk. The goals of OA management are pain reduction and improvement or preservation of mobility. A multidisciplinary approach to treatment is recommended.
NSAIDs are the most effective treatment for persons who experience the pain of osteoarthritis (OA), the most common chronic joint disease and a leading source of work disability. In many older patients, however, the risk of adverse effects with NSAIDs may outweigh the benefits. The GI risk is well known, and increased attention has been paid recently to the cardiovascular (CV) risk, in part because both rofecoxib and valdecoxib were withdrawn from the market several years ago.
At least 40 million adults in the United States report symptoms of OA, and as the population ages, the prevalence of the disease is expected to increase.1 The goals of OA management are pain reduction and improvement or preservation of mobility; no disease-modifying therapy is available, although extensive research is ongoing.
Considering the potential risks of NSAIDs, a multidisciplinary approach to treatment may be taken. Options include activity modification, analgesics, anti-inflammatories, local or topical treatments, dietary supplements (eg, glucosamine), and surgical interventions. The choice of treatment should be made on the basis of patient preference, disease severity, and medical comorbidities.
In this article, we provide an overview of the pathogenesis, diagnosis, and management of OA. We focus on recent data about the CV risk profile of NSAIDs and the efficacy of glucosamine.
PATHOGENESISRisk factors
The causes of primary OA probably are multifactorial: altered biomechanics, cytokine abnormalities, and genetic factors may combine to initiate a cascade of changes that become self-perpetuating as damage accumulates.2 The main risk factor for OA is age; more than 80% of persons older than 75 years are affected. However, OA is not a natural consequence of aging.
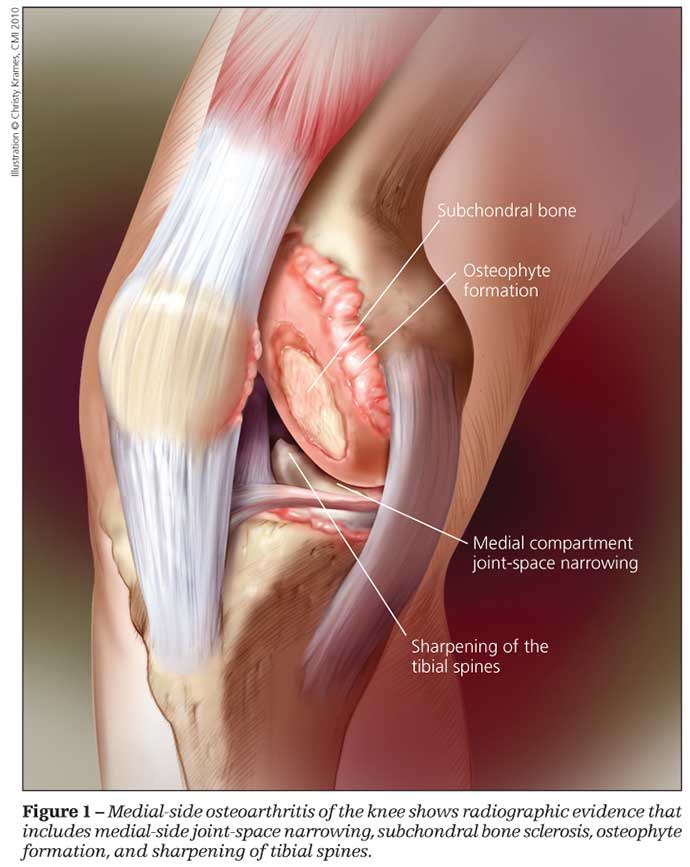
The knees are most frequently involved in primary OA (Figure 1), followed by the hips and hands.3 The mechanical forces experienced across weight-bearing joints clearly play a role; therefore, obesity is a strong risk factor for OA.4
OA of the hands and knees has a hereditary component, particularly in women.5 Family studies indicate that the heritable component of risk of OA may be 50% to 65%.5 Candidate genes have been identified as, among others, those coding for type II collagen, the vitamin D receptor, and the estrogen receptor.6
OA is twice as likely to develop in women as in men, especially after menopause. The finding of functional estrogen receptors on chondrocytes suggests that estrogen deficiency may mediate this association.7 In fact, hormone replacement therapy has been linked with decreased rates of OA in women.8
Low-impact exercises, including running, do not seem to contribute to OA in persons who have normal knee joints.9 However, participation in high-impact sports (eg, football and soccer) and previous joint injuries may contribute.
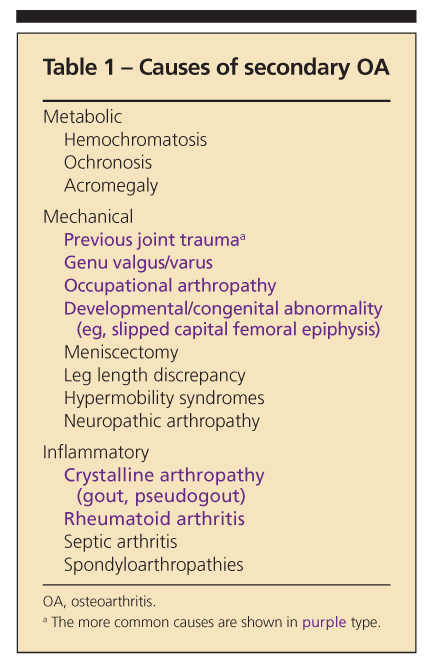
Secondary OA, which is less common than primary OA, may involve joints not frequently affected by primary OA. The causes of secondary OA include metabolic, mechanical, and inflammatory processes (Table 1).
DIAGNOSIS
Symptoms
Classic symptoms of OA include pain in the affected joint with or after activity. Pain at rest or night pain may occur with more advanced arthritis. Morning stiffness of 30 minutes or less duration is common; more prolonged periods of stiffness should prompt consideration of an inflammatory arthropathy as the cause.
There often is a poor correlation between symptoms and radiological findings. For example, two-thirds of women older than 55 years have radiological OA of the hands, but fewer than 20% are symptomatic.10
Physical examination
Findings on physical examination may include bony joint enlargement or the presence of an effusion. Pain may be reproduced with passive joint movement. Restricted range of motion should be noted. Crepitus may relate to intra-articular debris or irregularity in the cartilage contour. Joint locking usually indicates the presence of a loose body or a meniscal tear.
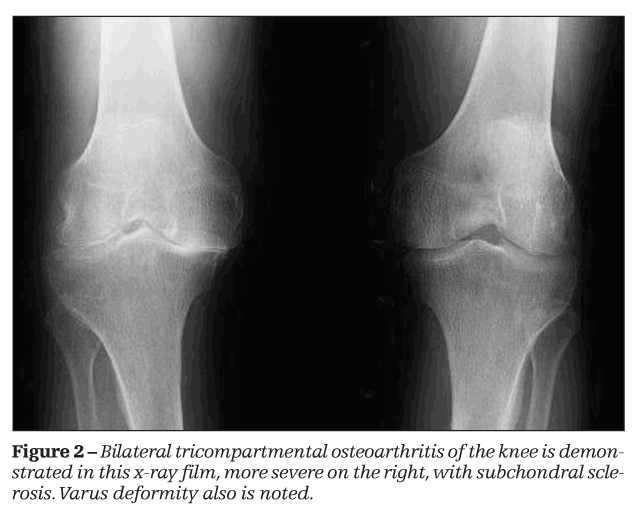
Muscle weakness, especially in the quadriceps, may be both a cause and an effect of OA of the knee (Figure 2). Gait evaluation may reveal a number of abnormalities, including varus or valgus deformities and leg length discrepancy.
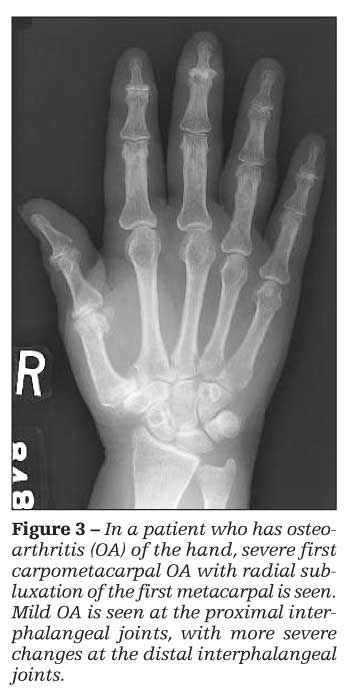
OA of the hand occurs most frequently in the distal interphalangeal (DIP) and proximal interphalangeal joints, as well as the first carpometacarpal joint (Figure 3). A subset of patients who have erosive changes of the DIP joints have a more inflammatory disease, erosive OA.
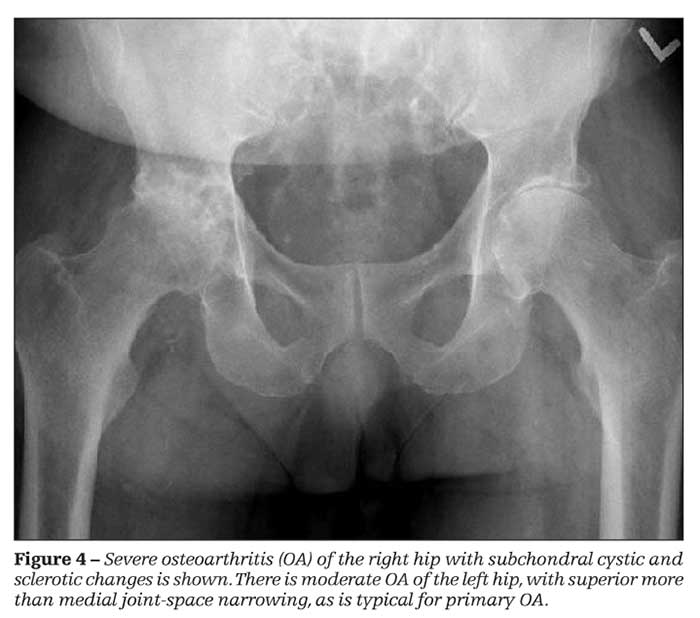
The pain that is associated with OA of the hip usually localizes to the groin (Figure 4). Lateral hip pain typically is nonarticular. Trochanteric bursitis should be excluded.
OA also is common in the posterior facet joints of the cervical and lumbar spine. First metatarsophalangeal arthritis is not uncommon, but arthritis of the ankle usually results from trauma.
Most shoulder pain relates to rotator cuff pathology rather than to true OA. However, OA may result from a chronic rotator cuff injury.
Imaging
If the signs and symptoms of OA are straightforward, radiological examinations are not necessarily indicated. They may be useful for monitoring progression if the joint involvement is unusual (eg, an ankle), to rule out other processes (eg, osteonecrosis), or if surgical intervention is being considered.
X-ray films of the knee should include weight-bearing views because other views underestimate the extent of joint-space narrowing. Osteophytes are a common early finding; subchondral sclerosis and joint-space narrowing occur later. Chondrocalcinosis becomes more common with age, especially in the knee, wrist, and pubic symphysis; it does not necessarily signify underlying calcium pyrophosphate dihydrate (CPPD) disease but should prompt its consideration. A normal x-ray film result does not exclude OA in a patient who has joint pain. MRI is much more sensitive but is not indicated unless ligamentous or meniscal damage that might warrant surgery is suspected.
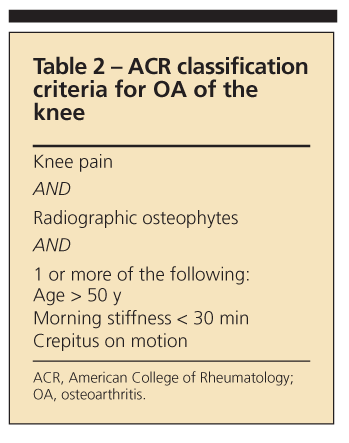
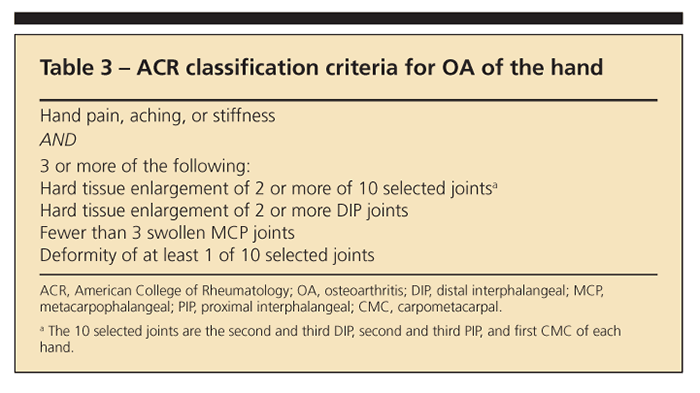
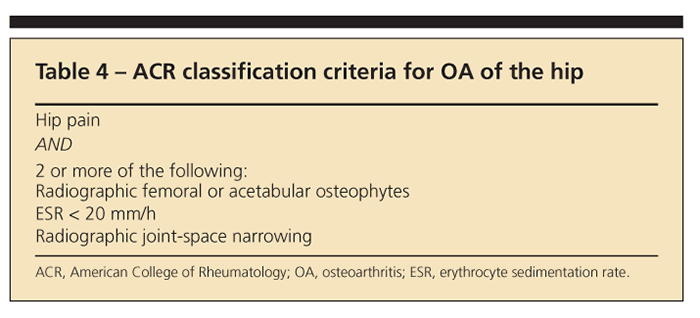
Tables 2, 3, and4 list the American College of Rheumatology classification criteria for OA of the
knee, hand, and hip, respectively. They provide a useful reference but were designed primarily for research rather than clinical practice.
Laboratory testing
Laboratory studies are not indicated for straightforward OA. The erythrocyte sedimentation rate and C-reactive protein level usually are normal. A low titer rheumatoid factor (RF) is common in older patients; an RF test should be ordered only if findings suggest an inflammatory process. Synovial fluid should be obtained if an effusion is present; the white blood cell count usually is less than 500/mm3, and crystals should be absent. A finding of CPPD crystals may indicate a patient who would benefit from receiving colchicine or NSAIDs.
TREATMENTLifestyle
Patients with OA of the knee should be encouraged to pursue low-impact exercises, such as walking, swimming, and water aerobics (Table 5). Weight loss may be the most important intervention in obese patients and may even improve pain in non–weight-bearing joints.
If performed consistently, exercises to strengthen the muscles around the knee may decrease pain. In one study, a structured physical therapy intervention improved pain and disability scores by 56% and decreased the rate of knee arthroplasty at 1 year from 20% to 5%.11
Ice may be effective at increasing range of motion, strength, and function in patients with OA of the knee.12 Knee braces and lateral wedge insoles may be helpful for some patients, but the long-term rate of compliance is low.13
Analgesics
Medical therapy usually is necessary, and evidence suggests that NSAIDs are slightly more efficacious than acetaminophen.14 However, most guidelines recommend acetaminophen as first-line
therapy because it has a more favorable adverse effects profile.15 Acetaminophen may be used at doses of up to 3 to 4 g/d (or 2 g/d in patients with liver disease), although the FDA is currently reviewing dosage recommendations and has highlighted the risk of liver damage in patients taking combinations of acetaminophen and narcotics.
An agent that often works well in combination with acetaminophen is tramadol, a unique analgesic that has weak opioid properties. Tramadol is associated with the same adverse effects that are well known with stronger narcotic agents; caution should be exercised in patients who are receiving selective serotonin reuptake inhibitors or serotonin-norepinephrine uptake inhibitors or who have a history of seizures.16
NSAIDs
With the withdrawal of rofecoxib and valdecoxib from the market, the remaining selective cyclooxygenase (COX)-2 inhibitor is celecoxib. This agent shows efficacy similar to that of the traditional NSAIDs, with reduced rates of GI adverse events (although the use of aspirin attenuates this benefit).17 In a retrospective study, however, the yearly myocardial infarction (MI) rate was significantly higher for celecoxib users compared with a historical control group (0.8% vs 0.52%; P = .04).18 A prospective trial demonstrated a 2- to 3-fold increase of MI in celecoxib users versus the placebo group.19

No placebo-controlled studies of CV risk with traditional NSAIDs have been published. Epidemiological data suggest that all NSAIDs increase the risk of CV events, with relative risks for first MI between 1.2 and 1.8. Most studies suggest that naproxen conveys the smallest increase in risk, and a few even suggest that it has a neutral CV risk profile.20
When given concomitantly, the traditional NSAIDs (but not celecoxib) inhibit the antiplatelet actions of aspirin.21 Overall, the CV safety profile of celecoxib appears to be better than that of the selective COX-2 inhibitors that were withdrawn from the market, similar to that of ibuprofen and diclofenac, and inferior to that of naproxen.22
Recommendations for NSAIDs include using the lowest effective dose for the shortest possible period. Lower doses of NSAIDs generally may be used to treat patients who have OA rather than those who have rheumatoid arthritis. Patients with GI risk factors (age older than 60 years, a history of a previous GI event, corticosteroid use, anticoagulation) should receive either a selective COX-2 inhibitor or a gastroprotective agent (proton pump inhibitor, misoprostol, double-dose H2 blocker), with a traditional NSAID. If an NSAID must be used, patients who have CV disease should receive naproxen.23 The American Geriatrics Society recommends avoiding NSAID use in all but the most highly selected older patients because of the risk of GI, renal, or CV toxicity.15
Topical/injection therapy
Many patients with OA experience persistent pain even though they are receiving oral analgesics. Topical NSAIDs (eg, diclofenac) provide a small but meaningful benefit for OA of the knee or hand, and the GI risk clearly is less than that of oral NSAIDs.24 Capsaicin may be effective for patients who can tolerate it, although it is less effective than topical NSAIDs.25
Corticosteroid injections often are useful for symptom control, especially in the knee. Controlled studies demonstrate a benefit for pain relief and physical function, although the superiority to placebo lasts only 4 weeks.26 The benefit of a corticosteroid injection in other joints, such as the shoulder or the thumb base, is less clear. There is no evidence that repeated injections cause joint damage, but the consensus is that a single joint should not be injected more than 4 times per year.
Viscosupplementation with hyaluronic acid is popular, although efficacy data remain mixed. One meta-analysis suggested a benefit similar to that of intra-articular corticosteroids but with the largest benefit being realized 1 to 3 months postinjection.27 Another meta-analysis noted that most studies were of poor quality and that the largest benefit was seen in the poorer quality studies.28
Supplements
Dietary supplements, particularly glucosamine, remain both popular and controversial. Some other supplements (eg, chondroitin and S-adenosylmethionine) are popular but less studied.
Glucosamine is well absorbed orally, but evidence for its benefit is mixed.29-31 Several preparations are available; glucosamine sulfate and hydrochloride are the most studied.
A recent review highlighted the difficulty in evaluating studies of glucosamine, noting their heterogeneity and frequently poor allocation concealment.32 In addition, the results of most independent studies have been negative while many industry-sponsored studies have shown benefit.
A Cochrane review of 25 studies involving 4963 patients reached similar conclusions: glucosamine produces a moderate reduction in pain and increase in function compared with placebo.32 The effect size was comparable to that of NSAIDs. The effect disappeared when only trials with adequate allocation concealment were considered. A subgroup analysis of studies using the Rotta formulation, a prescription glucosamine sulfate preparation from Europe, demonstrated moderate benefit.33
The highly publicized 2006 Glucosamine/chondroitin Arthritis Intervention Trial, which showed no benefit of glucosamine compared with placebo, was limited by a high placebo response rate and by the use of a glucosamine hydrochloride preparation, which prevented a direct comparison with other glucosamine knee OA trials that used a sulfate preparation. There was a trend toward benefit in pain scores for patients who had moderate to severe OA and were taking glucosamine.34 The Joints on Glucosamine study, the most recent randomized evaluation of glucosamine for OA, used MRI of OA progression in the knee as an end point and similarly found no benefit to glucosamine (hydrochloride) use.35
Overall, these data indicate that consensus about the efficacy of glucosamine is lacking. Because adverse events are rare, a trial of glucosamine sulfate, 1500 mg/d, may be recommended for symptomatic patients. Patients should purchase from a reputable manufacturer and, if no benefit is evident within 6 weeks, consider discontinuing the drug.
Surgery
Symptoms persist in some patients even though they have received maximal medical treatment. In these cases, referral to an orthopedic surgeon is reasonable.
TAKE-HOME POINTS
Key points to consider in OA management include the following:
•Straightforward cases of OA do not require imaging or blood tests unless the involved joint is unusual, an alternative diagnosis is being considered (eg, osteonecrosis), or surgical intervention is being considered.
•Acetaminophen, weight loss, exercise, and physical therapy should not be overlooked as key interventions. They may obviate the need for medications that have greater risks (NSAIDs, opioid analgesics) or for surgery.
•NSAIDs are effective for OA, but patient-specific risk factors for adverse events must be considered carefully and their use in older patients or those who have known coronary disease must be weighed carefully.
•If a trial of glucosamine is recommended, consider using the sulfate preparation, and if no benefit is evident after 6 to 8 weeks, the drug probably can be stopped.
References:
References1. Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3-25.
2. Mankin HJ, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg. 1970;52A:424-434.
3. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
4. Grotle M, Hagen KB, Natvig B, et al. Obesity and osteoarthritis of knee, hip, and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132.
5. Spector T, Cicuttini F, Baker J, et al. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312:940-943.
6. Loughlin J. Genetic epidemiology of primary osteoarthritis. Curr Opin Rheumatol. 2001;13:111-116.
7. Richmond RS, Carlson CS, Register TC, et al. Functional estrogen receptors in adult articular cartilage: estrogen replacement therapy increases chondrocyte synthesis of proteoglycans and insulin-like growth factor binding protein 2. Arthritis Rheum. 2000;43:2081-2090.
8. Nevitt MC, Cummings SR, Lane NE, et al. Association of estrogen replacement therapy with the risk of osteoarthritis of the hip in elderly white women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1996;156:2073-2080.
9. Buckwalter JA, Martin JA. Sports and osteoarthritis. Curr Opin Rheumatol. 2004;16:634-639.
10. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, et al. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) [published correction appears in Ann Rheum Dis. 2005;64:1248]. Ann Rheum Dis. 2005;64:682-687.
11. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 2000;132:173-181.
12. Brosseau L, Yonge KA, Robinson V, et al. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev. 2003;(4):CD004522.
13. Brouwer RW, Jakma TS, Verhagen AP, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2005;(1):CD004020.
14. Pincus T, Koch GG, Sokka T, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2001;44:1587-1598.
15. American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331-1346.
16. Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006; (3):CD005522.
17. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954-959.
18. Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ. 2002;325:619.
19. Solomon SD, McMurray JJ, Pfeffer MA, et al; Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080.
20. GarcÃa-RodrÃguez L, González-Pérez A. Long-term use of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction in the general population. BMC Med. 2005;3:17.
21. Capone ML, Tacconelli S, Sciulli MG, et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 2004;109:1468-1471.
22. Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302-1308.
23. Osteoarthritis of the knees. National Guideline Clearinghouse. http://www.guidelines.gov/summary/summary.aspx?view_id=1&doc_id=11086. Accessed January 5, 2010.
24. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (Pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
25. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
26. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869.
27. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.
28. Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172:1039-1043.
29. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251-256.
30. Rindone JP, Hiller D, Collacott E, et al. Randomized, controlled trial of glucosamine for treating osteoarthritis of the knee. West J Med. 2000;172:91-94.
31. Rozendaal RM, Koes BW, van Osch GJ, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148:268-277.
32. Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum. 2007;56:2267-2277.
33. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.
34. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
35. Kwoh CK, Roemer FW, Hannon MJ, et al. The Joints On Glucosamine (JOG) study: a randomized, double-blind, placebo-controlled trial to assess the structural benefit of glucosamine in knee osteoarthritis based on 3T MRI. Presentation No. 1942. Presented at: the ACR/ARHP Annual Scientific Meeting; 2009; Philadelphia.




