Plaque Psoriasis: Cosentyx Continues to Come Out on Top
Novartis announced results from the CLEAR study demonstrating Cosentyxâ„¢ (secukinumab) improved skin clearance at Week 16 in significantly more moderate-to-severe plaque psoriasis patients compared to Stelara®* (ustekinumab), a widely used biologic.
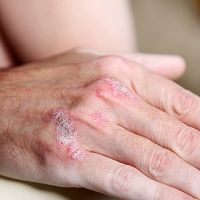
Novartis announced results from the CLEAR study demonstrating Cosentyx™ (secukinumab) improved skin clearance at Week 16 in significantly more moderate-to-severe plaque psoriasis patients compared to Stelara®* (ustekinumab), a widely used biologic.
Findings were presented in a late-breaking research session at the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco.
Cosentyx is the first and only FDA-approved interleukin-17A (IL-17A) antagonist to treat adult patients with moderate-to-severe plaque psoriasis.
Andrew Blauvelt, MD, MBA, President of the Oregon Medical Research Center, CLEAR’s lead author, conducted a 52-week, multicenter, randomized, double-blind study, a head-to-head Phase IIIb study initiated with Cosentyx, comparing the efficacy, long-term safety and tolerability of Cosentyx (secukinumab) versus Stelara (ustekinumab), in patients with moderate-to-severe plaque psoriasis.
The secondary endpoint found 50% of Cosentyx patients achieved a Psoriasis Area Severity Index (PASI) response of 75 at Week 4 compared to 20.6% of Stelara patients (P<0.0001).
COSENTYX is contraindicated in patients with a previous serious hypersensitivity reaction to secukinumab or to any of the excipients.
The most common adverse reactions (>1%) were reported as nasopharyngitis, diarrhea, and upper respiratory tract infection.
Blauvelt commented, “As a clinician I have seen first-hand the impact that moderate-to-severe plaque psoriasis can have on patients’ lives and the frustration some feel when they are unable to achieve clear or near clear skin.”
Christi Shaw, US Country Head, President of Novartis Corporation and Novartis Pharmaceuticals Corporation said in a news release, “The findings from the CLEAR study demonstrate that Cosentyx helps a majority of patients relieve the thick, red, scaling and extensive plaques that are common in those living with psoriasis. The data reinforce the importance of Cosentyx as a beneficial new treatment option, offering hope to psoriasis patients so they can take control of this often isolating disease.”