Providers Express Difficulties in Transitioning to Biosimilars
Providers identified an increased administrative workload and inadequate biosimilar device training as pain points during a mandatory switch to a biosimilar.
Chiara Gasteiger, PhD
Credit: University of Auckland

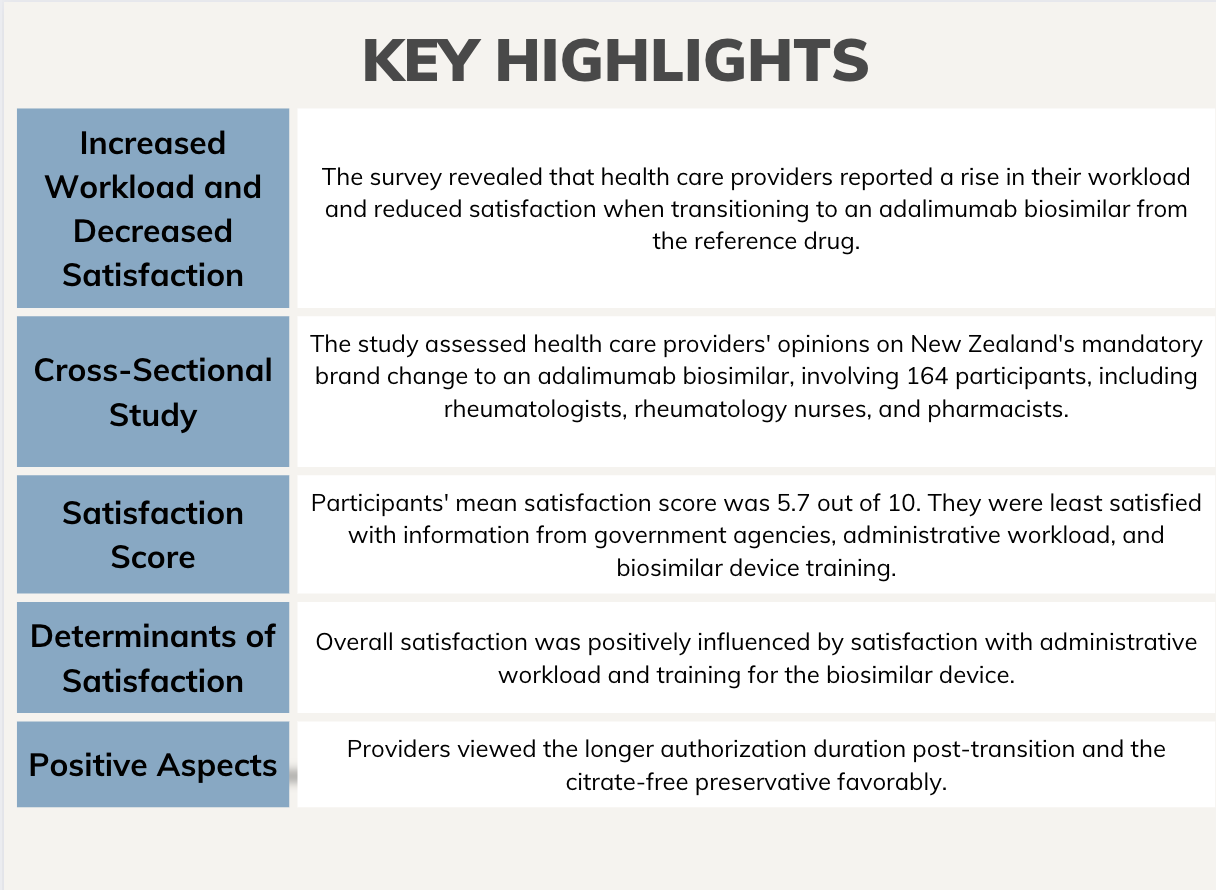
Results of a recent survey revealed health care providers reported an increased workload and less satisfaction with an adalimumab biosimilar following a mandatory switch from the reference drug. The survey also indicated providing training for the device, functioning authorization processes, and a high-quality patient support program may improve these experiences, according to data published in Open Rheumatology.1
“Transitioning patients to biosimilars has become common to reduce costs and improve access,” wrote Chiara Gasteiger, PhD, affiliated with the School of Medicine and Department of Psychological Medicine at the University of Auckland, and colleagues. “However, it is unclear how the transition process impacts health care providers at the frontline of the brand change.”
Although switching to a biosimilar is often used to decrease the healthcare system’s financial burden, some providers have voiced concerns about the drug’s efficacy, safety, quality, and interchangeability, which may inadvertently impact a patient’s confidence in the switch. They are also challenged with ensuring their patients are supported during the transition period, a potentially difficult time for clinically stable patients worried about a loss of disease control.2
A cross-sectional study assessed health care providers’ opinions of Aotearoa New Zealand’s nationwide mandatory brand change to an adalimumab biosimilar using online survey data from 164 participants, including 39 rheumatologists, 16 rheumatology nurses, and 109 pharmacists. The survey, which was conducted between November 2022 and February 2023, evaluated their satisfaction with both supply and logistics, support, education and information, and administrative workload to determine what did and did not go well during the transition period. Demographic data were also collected, including age, sex, ethnicity, employment status, and the number of years practicing, as well as any previous experience with a biosimilar brand change.
The sample was predominantly New Zealand European (67%), female (60%), and employed fulltime (73%). Providers had been practicing for an average of 22.5 years.
Among the participants, the mean satisfaction score, on a scale of 0 – 10, was 5.7. Providers reported being least satisfied with the information provided by government agencies, the administrative workload, and the training offered for the biosimilar device. Additionally, their satisfaction with adalimumab’s efficacy, safety, and device quality, and the availability of alcohol wipes, sharps bins, and patient support was lower post-transition.
The predictors of overall satisfaction with the transition were satisfaction with administrative workload (B = .37, P <.001) and training for the device (B = .20, P = .020). The transition to the biosimilar was complicated due to the loss of a patient support program, poor communication between providers, and a poorly implemented initial authorization process, with 33% reporting an increased workload due to authorization issues. Ninety providers stated their workload increased during transition and 46% reported needing to provide additional counseling and education to their patients. However, providers viewed the longer authorization duration post-transition and the citrate-free preservative, designed to reduce pain during injection, favorably.
Investigators noted limitations, including receiving responses from only 3% of practicing pharmacists, which may impact generalizability. Further, there is a possibility providers who experienced a negative transition period were more likely to respond to the survey. However, the sample did include 61% of practicing rheumatologists and 62% of practicing rheumatology nurses. The experiences of providers may not be generalizable to other healthcare systems, as this study focused on brand change in Aotearoa New Zealand. Future research should concentrate on simplifying the transition process and consider the providers’ experiences.
“Despite differences in roles and responsibilities between rheumatologists, pharmacists, and rheumatology nurse specialists, the findings demonstrate the importance of ensuring the biosimilar brand package is comparable with the originator and that the administrative and educational process is simple for all health care providers involved,” investigators concluded. “It is crucial to continue learning from and sharing experiences of biosimilar brand changes globally, to ultimately understand how to streamline the transition process and reduce the additional workload for providers.”
References
- Gasteiger C, Lobo M, Wong LS, Murdoch R, Dalbeth N. Health Care Providers' Experiences of a Mandatory Nationwide Transition to an Adalimumab Biosimilar [published online ahead of print, 2023 Oct 1]. ACR Open Rheumatol. 2023;10.1002/acr2.11617. doi:10.1002/acr2.11617
- Gasteiger C, Jones AS, Kleinstäuber M, et al. Effects of message framing on patients’ perceptions and willingness to change to a biosimilar in a hypothetical drug switch. Arthritis Care Res (Hoboken) 2020; 72: 1323–30.