Article
Quantitative Joint Assessment to Improve RA Outcomes
The RA Joint Count is a validated tool for assessing rheumatoid arthritis. This article reviews a uniform procedure for instructing patients, identifying joint landmarks, and examination techniques.
ABSTRACT: Quantitative assessment of joint swelling and tenderness, essential for rheumatoid arthritis (RA) clinical research, also improves outcomes in routine clinical patient care. Both old and new RA classification criteria involve careful joint assessment, and the results of several studies indicate that performing quantitative joint counts improves patient care. We synthesized the published information and developed a video CME program to provide physicians and other health care professionals with training on performing quantitative joint examinations. The RA Joint Count, which includes 6 joint areas for a total of 28 joints, involves a uniform procedure that includes specific instructions to the patient, precise identification of anatomical joint landmarks, and defined joint examination techniques. This validated tool provides important information for assessing severity and outcomes in RA. (J Musculoskel Med. 2011;28:79-84)
Quantitative assessment of joint swelling and tenderness is essential for rheumatoid arthritis (RA) clinical research. It also has been shown to improve outcomes in routine clinical care of patients with RA.
RA is a common problem, affecting 1.3 million persons in the United States and millions more worldwide, and diagnosis and management can be challenging.1,2 In this article, we discuss the need for joint assessment in patient evaluation and demonstrate how use of the RA Joint Count tool can facilitate effective evaluation for improved outcomes.
BACKGROUND
The American College of Rheumatology (ACR) established criteria for the classification of RA in 1987 and with the European League Against Rheumatism (EULAR) revised the criteria in 2010 to permit earlier diagnosis and to incorporate anti–cyclic citrullinated peptide antibody testing.3,4 Both the old and the new RA criteria involve careful joint assessment. In addition, the ACR established criteria for improvement in RA (the ACR 20, 50, and 70 responses)5 and the EULAR developed RA response criteria based on the Disease Activity Score (DAS).6-8 Both the ACR improvement criteria and the DAS include the number of joints that are swollen and tender.
Studies also have emphasized the value of quantitative joint counts for the routine clinical care of patients with RA. In the Tight Control for Rheumatoid Arthritis (TICORA) study, a “treat to target” strategy was used.9 The TICORA investigators compared usual care with more intensive care of patients with RA who have active disease. The usual-care group was evaluated every 3 months, and no quantitative measures of disease activity were used. In contrast, the intensive-treatment group received therapy that was adjusted using a predefined algorithm based on the DAS score.
Patients who received the intensive-therapy regimen achieved a lower DAS score more rapidly and sustained the lower DAS score longer than patients with RA treated in the usual fashion. There was faster improvement in the intensive-regimen group in nearly every parameter that reflects rheumatoid disease activity, including (1) tender and swollen joint counts; (2) Health Assessment Questionnaire score; (3) pain scores; (4) erythrocyte sedimentation rate (ESR); and (5) Short-Form-12 score, which measures quality of life.
Similar results were obtained in the Behandel Strategien (“treatment strategies”) and Computer-Assisted Management in Early Rheumatoid Arthritis (CAMERA) studies.10,11 In both studies, patients with early RA were treated with therapies adjusted on the basis of disease activity measurements. The CAMERA study compared usual care with care that used a computer algorithm to adjust therapy; the algorithm included the number of swollen joints and tender joints, the ESR, and a physician global assessment of disease severity.11 As in the TICORA study, patients with RA treated to target had better immediate and long-term outcomes than those receiving usual care. Together, the results of these studies indicate that performing quantitative joint counts improves the care of patients with RA.
VIDEO TRAINING PROGRAM
Given the importance of the quantitative joint examination for clinical research studies and for RA patient care, we reviewed the literature related to joint examination techniques.12-14 We synthesized the published information and developed a video CME program to provide physicians and other health care professionals with training on performing quantitative joint examinations.
Assessment of articular tenderness in RA initially was proposed by Ritchie and associates13 in 1964. The standardized RA Joint Count, adapted from the 1989“simplified twenty-eight–joint quantitative articular index” described by Fuchs and colleagues,14 is used in the ACR improvement criteria and in the DAS. It can be performed effectively and efficiently using uniform procedures, precise identification of anatomical landmarks, and defined joint examination techniques.12,15
The RA Joint Count includes 6 joint areas, with a total of 28 joints: the right and left proximal interphalangeal (PIP) joints, metacarpophalangeal (MCP) joints, wrists, elbows, shoulders, and knees. Each joint is assessed by inspection and palpation. With practice, the RA Joint Count takes about 2 to 3 minutes to perform. To provide survey consistency, the joint count involves the 3 primary elements noted above: (1) a uniform procedure that includes specific instructions to the patient, (2) precise identification of anatomical joint landmarks, and (3) defined joint examination techniques.
To allow for sufficient joint inspection, the physician should ask the patient to wear a standard gown and sit on the front end of the examination table. The patient should be provided with the following instructions: “I am going to examine various joints for swelling and tenderness. Please say yes or no if there is tenderness when I press a specific joint.”
In RA, joint swelling and tenderness are caused by inflammation of the joint synovium, increased synovial fluid production, and periarticular swelling in the joint capsule and surrounding tissues. Visual clues of joint swelling include enlargement of the joint soft tissue, skin stretching with loss of folds and furrows, and red or blue skin color changes around the joint.
After inspection, each joint is examined by palpation using the thumbs and index fingers. Joint swelling may be detected by finding tissue sponginess and ballotting the joint for increased synovial fluid. Identifying anatomical landmarks of specific joints and comparing joints on one side of the body with those on the other side are helpful in assessment of joint swelling.
Note that RA joint and soft tissue structural abnormalities may cause joint malalignment, which may influence the assessment for swelling. Muscles and subcutaneous tissue can atrophy, making the joints appear more prominent. Adipose tissue may be present near joints and is related, in part, to body size; it should not be confused with inflammation. Joints that have severe deformity or are fused should be noted and should not be included in the scoring for swelling.
During joint palpation, tenderness is determined primarily by applying sufficient pressure to the joint line to cause blanching of the examiner's fingernail bed. The patient is asked, “Is that tender?”
Joint tenderness also is assessed by moving joints through their respective ranges of motion. Limited range of motion may be a sign of joint inflammation; this should be interpreted in the context of simultaneous assessment of a patient's pain reaction to joint movement, such as facial grimacing or joint withdrawal.
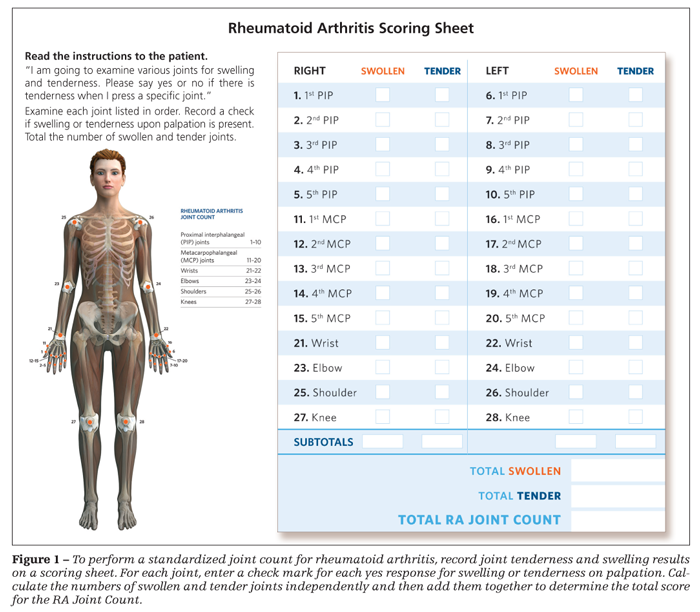
Joint tenderness and swelling results should be recorded on a score sheet, such as that shown in Figure 1. The numbers of swollen and tender joints are calculated independently and then added together to determine the total score for the RA Joint Count. As part of the quantitative assessment of disease activity, collecting the physician's global assessment of disease activity also is helpful.
AREAS OF EXAMINATION
Following are instructions for examination of each of the 28 joints that are part of the RA Joint Count.
Hands
•Interphalangeal (IP) joint 1 and PIP joints 2-5. The patient is instructed to make a fist and then extend the fingers to make the palm as flat as possible, which allows for inspection of joint range of motion and places the 4 PIP joints and the first IP joint in a neutral position. Thin horizontal skin folds present over the joints decrease with joint flexion.
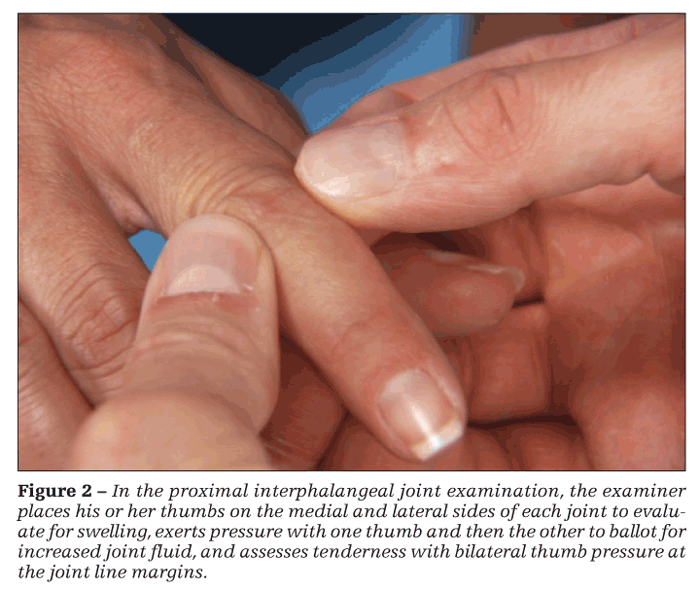
The joints are flexed to 30°. The examiner places his or her thumbs on the medial and lateral sides of each PIP joint to examine for swelling (Figure 2). Next, he exerts pressure with one thumb and then the other to ballot for increased joint fluid. A 4-finger approach also may be used for ballottement. One thumb and index finger are placed on the ventral and dorsal sides of the patient's joint and the other thumb and index finger on the sides at the joint line. Tenderness is assessed with bilateral thumb pressure at the joint line margins.
•MCP joints 1-5. The MCP joints are evaluated by inspection with the patient's fingers fully extended using a procedure similar to that used for the PIP joints. This position reduces the transverse and longitudinal arches of the hand and the prominence of the second and third MCP joints. Each of the MCP joints is examined visually for clues. Usually, there are thin horizontal skin folds over the joints that stretch with flexion. The furrows between the MCP joints decrease with swelling.
The MCP joints are flexed to 30°. The examiner presses his thumbs inwardly in a horizontal plane on the sides at each joint line. The patient's thumb MCP joint is palpated with the hand rotated laterally to detect sponginess of tissues resulting from inflammation.
The hand is returned to the neutral position to examine MCP joints 2-5. Synovial fluid movement is felt with pressure applied on the sides of the MCP joints. Skin, subcutaneous fat between the metacarpals, and ligamentous structures add to the sensation of palpation and must be accounted for when judging joint swelling. Tenderness of each MCP joint along the medial and lateral joint line margin is determined by applying thumb pressure that blanches the examiner's nail.
Several additional anatomical points should be considered in the MCP and PIP joint examination. Adipose tissue may be present on the dorsum of the hand; it is related, in part, to body size and should not be confused with inflammation. Bony changes may occur (RA erosions or osteophytes resulting from osteoarthritis). RA swan neck, boutonnire, and MCP ulnar deviation deformities do not necessarily indicate swelling or tenderness. The intrinsic muscles of the hands between the metacarpals may become atrophied in RA, increasing the furrows between the metacarpals in the dorsal hand. The furrows may be lost with MCP joint swelling.
Wrists
The wrist joint is divided by septae into radiocarpal and inferior radioulnar cavities. Wrist inflammation may occur in the articular synovium and in the dorsal and volar synovial sheaths. These sheaths surround the tendons, which connect the forearm muscles to the fingers and extend to midway between the wrist and the MCP joints.
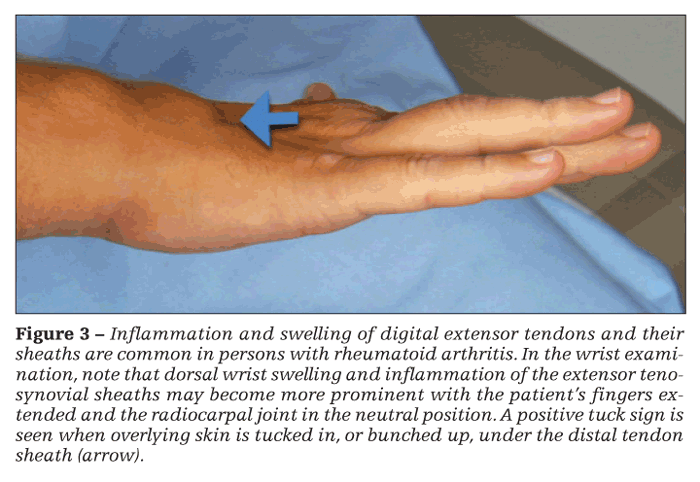
Assessment of the wrist should be performed by observing the joint in both the neutral and flexed positions. Wrist swelling is detected with thumb palpation applied dorsally along the joint line and at the ulnar styloid. Dorsal wrist swelling and inflammation of the extensor tenosynovial sheaths may become more prominent with the patient's fingers extended and the radiocarpal joint in the neutral position. Overlying skin that is tucked in, or bunched up, under the distal tendon sheath is a positive tuck sign (Figure 3).
Volar radiocarpal and extrinsic finger flexor tendon swelling on the palmar side of the joint also may be detected with palpation. Deformity of the wrist with rotatory subluxation causes prominence of the ulnar styloid region, making assessment for swelling more difficult. Also, normally there is a fat pad adjacent to the ulnar styloid that should not be scored as swelling. Joint tenderness is elicited by palpating over the radiocarpal joint line dorsally and at the ulnar styloid.
Elbows
The elbows are inspected with the joint brought to full extension. Swelling is evident visually as a loss of the medial and lateral recesses on either side of the ulna. With the patient's elbow flexed and extended, the examiner uses his thumb and index finger to palpate the lateral recess between the lateral epicondyle of the humerus and the head of the radius and the medial recess between the medial epicondyle and the ulna. Swelling is detected as a spongy sensation or bulging fluid. To determine tenderness, pressure is applied to the elbow along the lateral joint line with the thumb pad.
Posterior swelling in the olecranon bursa, which lies superficially over the olecranon, is not considered true elbow joint swelling. Elbow subcutaneous nodules also are not considered swelling. The findings are recorded on the basis of the presence of swelling in the medial and lateral recesses of the elbow and tenderness along the joint line on palpation.
Shoulders
Evaluation of shoulder swelling is challenging because of the musculature overlying the joint. In addition, the shoulders may not be similar in size-the shoulder of the dominant hand often is larger.
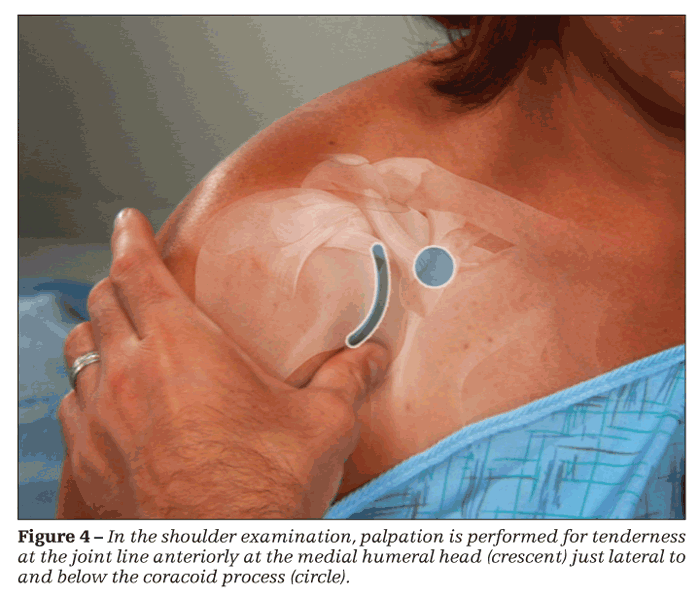
The patient is assisted in abducting the arm 50° from the body laterally. Unlike in other joints, tenderness in the shoulder is not determined principally with palpation but rather with the patient's report of pain with abduction. The examiner detects synovitis and synovial effusions of the shoulder by using the thumb pads at the joint line anteriorly at the medial humeral head just lateral to and below the coracoid process (Figure 4). The patient's arm should be next to the body with the forearm resting on his leg. The examiner ballots the shoulder joint for fluid with pressure applied posteriorly moving fluid anteriorly, making
it easier to detect. Shoulder tenderness also may be elicited by applying thumb pressure to the shoulder joint line anteriorly.
Knees
The knee, the largest joint in the body, is involved in RA frequently. Inspection starts with the patient lying flat with the hip externally rotated to 60° and the knee slightly flexed. Then, joint range of motion with flexion and extension is assessed by comparing one knee with the other.
There are recesses medial and lateral to the patella along the joint line. Suprapatellar and infrapatellar bursae are present above and below the knee. Each of these recesses and bursae may become swollen with synovial proliferation and increased joint fluid.
In more than 90% of persons, the suprapatellar bursa communicates with the knee joint and fluid there may be moved to the joint cavity. The examiner detects swelling by placing a hand on the suprapatellar region and applying downward pressure. Palpating with the thumb and index finger medially and laterally below the patella at the joint margin detects movement of fluid. Ballottement of the patella also may help determine the presence of fluid.
Tenderness in the knee is assessed by applying thumb pressure over the medial joint line, which is proximal to the anserine bursa and is felt as a groove. Fat pads above and below the knee in the suprapatellar and infrapatellar regions may be prominent, depending, in part, on body size. Knee swelling and tenderness are recorded on the scoring sheet.
SUMMARY
The RA Joint Count is a validated tool that provides important information for assessing severity and outcomes in RA. With the use of uniform procedures, precise identification of anatomical landmarks, and defined joint examination techniques, it can be performed effectively and efficiently to improve outcomes for patients with RA.
References
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part I. Arthritis Rheum. 2008;58:15-25.
2. Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861-1874.
3. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315-324.
4. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-2581.
5. Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology: preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727-735.
6. van Gestel AM, Prevoo ML, van 't Hof MA, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis: comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39:34-40.
7. van der Heijde DM, van 't Hof MA, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579-581.
8. Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-48.
9. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263-269.
10. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381-3390.
11. Bakker MF, Jacobs JW, Verstappen SM, Bijlsma JW. Tight control in the treatment of rheumatoid arthritis: efficacy and feasibility [published correction appears in Ann Rheum Dis. 2008;67:140]. Ann Rheum Dis. 2007;66(suppl 3):iii56-60.
12. Keystone E, Weber D. Physical examination. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatoid Arthritis. Philadelphia: Mosby-Elsevier; 2009:279-284.
13. Ritchie DM, Boyle JA, McInnes JM, et al. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968;37:393-406.
14. Fuchs HA, Brooks RH, Callahan LF, Pincus T. A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum. 1989;32:531-537.
15. Standring S, ed. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 39th ed. Edinburgh: Elsevier Churchill Livingstone; 2005.




