Ramosetron Shows Long-Term Efficacy in IBS-D without Alosetron's Adverse Side Effects
In a clinical review published in Clinical and Experimental Gastroenterology, researchers from Iwate Medical University in Japan evaluated the long-term safety and efficacy of the novel serotonin-receptor agonist ramosetron in patients suffering from diarrhea-predominant irritable bowel syndrome (IBS-D).

In a clinical review published in Clinical and Experimental Gastroenterology, researchers from Iwate Medical University in Japan evaluated the long-term safety and efficacy of the novel serotonin-receptor agonist ramosetron in patients suffering from diarrhea-predominant irritable bowel syndrome (IBS-D).
For their “Long-term Efficacy and Safety of Ramosetron in the Treatment of Diarrhea-Predominant Irritable Bowel Syndrome” research, Toshimi Chiba, MD, and colleagues examined data on ramosetron from two double-blind, placebo-controlled, parallel-group studies with a total of 957 IBS-D patients and a 12-week randomized controlled trial of 539 IBS-D patients.
In the first double-blind study, once-daily doses of 5 or 10 micrograms of ramosetron “increased the monthly responder rates of “patient-reported global assessment of relief of IBS symptoms” compared to placebo,” while the second double-blind trial found a once-daily 5 microgram dose of ramosetron “was effective and well tolerated in the treatment of abdominal pain, discomfort, and altered bowel habits,” the authors wrote.
A similar positive response to once-daily 5-microgram ramosetron treatment was discovered in IBS-D patients enrolled in the 12-week randomized study, as “relief of abdominal pain or discomfort was reported by 46 percent of patients with ramosetron versus 33 percent with placebo (P=0.005), and improved abnormal bowel habits were reported by 44 percent with ramosetron compared to 24 percent with placebo (P<0.001),” the researchers reported. Additionally, results of the trial’s post-marketing survey demonstrated “long-term efficacy for overall improvement of IBS symptoms” up to 52 weeks, according to the authors. (Table on next page)
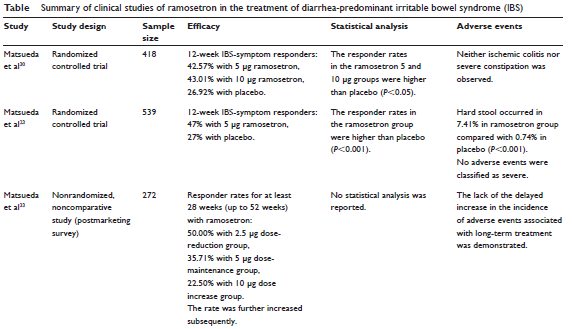
Comparing adverse events in IBS-D patients receiving ramosetron to those treated with the more potent serotonin-receptor agonist alosetron, the researchers found the incidence of mild or moderate constipation with ramosetron was 5.2 percent, which was significantly lower than alosetron’s 29 percent constipation incidence. In addition, ischemic colitis and severe constipation were reported with alosetron use, but neither of those adverse side effects was observed in IBS-D patients treated with ramosetron.
Given that ramosetron “has been useful in attaining relief of abdominal pain or discomfort and improvements in abnormal bowel habits” — and in contrast to alosetron, “adverse events such as abdominal distension, constipation, and hard stool, and ischemic colitis (are) unlikely to be caused by ramosetron” — the authors concluded that the serotonin-receptor agonist “would be the candidate of first choice for treating IBS-D clinically,” though they noted that “further studies to evaluate the long-term efficacy and safety of ramosetron are warranted in the form of randomized controlled trials.”