Statins Do Double Duty Against Stroke
A new study suggests that inpatient statin use is linked to improved outcomes after ICH, and that stopping statins during hospitalization is associated with worse outcomes.

A new study suggests that inpatient statin use is linked to improved outcomes after ICH, and that stopping statins during hospitalization is associated with worse outcomes. PSK9 inhibitors are a new class of injectable antihyperlipidemics that have been shown to lower LDL and may also decrease risk of cardiovascular events, including stroke. A study has suggested that evolocumab, a new PSK9 inhibitor, plus standard therapy reduces LDL by 61% and cardiovascular events by about half, compared to standard therapy alone. Another study has suggested that alirocumab, also a PSK9 inhibitor, reduces LDL by 62% and cardiovascular risk by about half, compared to placebo. • Both PSK9 agents were linked to increased rates of neurocognitive disorders

Inpatient statin use has been strongly linked to improved survival and a better discharge disposition after ischemic stroke. Conflicting evidence exists about the use of statins in the acute setting and the risk of brain hemorrhage, especially among patients with a history of intracranial hemorrhage. A retrospective cohort study was conducted involving 3481 patients admitted with ICH over a 10-year period (January 2002 to December 2011) to 20 hospitals within the Kaiser Permanente health system in Northern California . Data on statin use before and during admission were abstracted from the electronic medical record; patients were followed for 30 days from admission or until death. Flint AC, Conell C, Rao VA, et al. Effect of statin use dring hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol. 2014; 71(11): 1364-1371. Doi: 10.1001/jamaneurol.2014.2124 More information, here.

After controlling for confounders, patients hospitalized with ICH who received inpatient statins were more likely than non-users to be alive at 30 days (OR 4.25 [95% CI 3.46-5.23; p
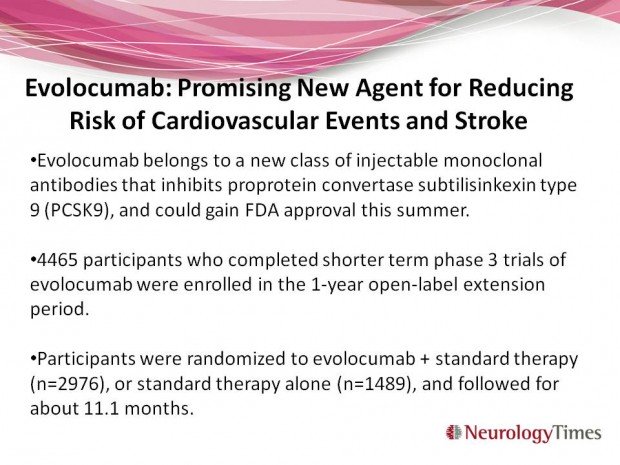
Evolocumab belongs to a new class of injectable monoclonal antibodies that inhibits proprotein convertase subtilisinkexin type 9 (PCSK9), and could gain FDA approval this summer. 4465 participants who had completed shorter term phase 3 trials of evolocumab were enrolled in the one year open-label extension period. Participants were randomized to evolocumab plus standard therapy (n=2976), or standard therapy alone (n=1489), and followed for about 11.1 months Sabatine MS, GIugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in educing lipids and cardiovascular events. NEJM 2015 Apr 16;372(16):1500-1509. Epub 2015 Mar 15. Doi: 10.1056/NEJMoa1500858 More information, here.

Compared to standard therapy alone, evolocumab plus standard therapy reduced LDL by 61% (p

Alirocumab also belongs to the new class of injectable monoclonal antibodies of PCSK9 inhibitors, and could also win FDA approval this summer. Randomized, placebo-controlled, double-blind phase 3 trial that took place in 27 countries; enrolled 2341 patients at high risk for cardiovascular events, with LDL levels of 70 mg/dL and above, and already receiving statins at the maximum tolerated dose. Participants were randomly assigned to alirocumab (n=1553) or placebo (n=788) every 2 weeks for 78 weeks. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;16;372:1489-1499. Epub 2015 Mar 15. More information, here.
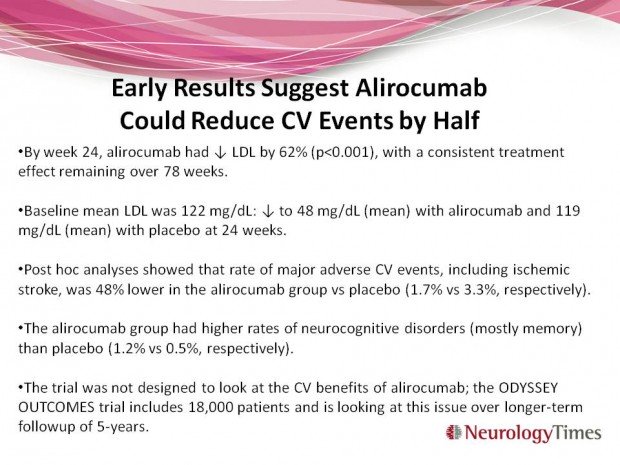
By week 24, alirocumab had lowered LDL by 62% (p

A new study suggests that inpatient statin use is linked to improved outcomes after ICH, and that stopping statins during hospitalization is associated with worse outcomes. PSK9 inhibitors are a new class of injectable antihyperlipidemics that have been shown to lower LDL and may also decrease risk of cardiovascular events, including stroke. A study has suggested that evolocumab, a new PSK9 inhibitor, plus standard therapy reduces LDL by 61% and cardiovascular events by about half, compared to standard therapy alone. Another study has suggested that alirocumab, also a PSK9 inhibitor, reduces LDL by 62% and cardiovascular risk by about half, compared to placebo. Both PSK9 agents were linked to increased rates of neurocognitive disorders.
A new study suggests that inpatient statin use is linked to improved outcomes after intracranial hemorrhage. And 2 new studies suggest that a new class of injectable antihyperlipidemics, the PSK9 inhibitors--which have been shown to lower LDL-- may decrease cardiovascular events, including stroke.
Details of those studies appear in the following slides. Take home points include:
Inpatient statin use is linked to improved outcomes after ICH, and that stopping statins during hospitalization is associated with worse outcomes.
PSK9 inhibitors are a new class of injectable antihyperlipidemics that have been shown to lower LDL and may also decrease risk of cardiovascular events, including stroke
A study has suggested that evolocumab, a new PSK9 inhibitor, plus standard therapy reduces LDL by 61% and cardiovascular events by about half, compared to standard therapy alone
Another study has suggested that alirocumab, also a PSK9 inhibitor, reduces LDL by 62% and cardiovascular risk by about half, compared to placebo
Both PSK9 inhibitors were linked to increased rates of neurocognitive disorders