Symptom-Targeting Non-Pharmacological Approaches Individualize Fibromyalgia Treatment
Personalizing non-pharmacological treatment for patients with fibromyalgia using a targeted symptom approach may improve outcomes.
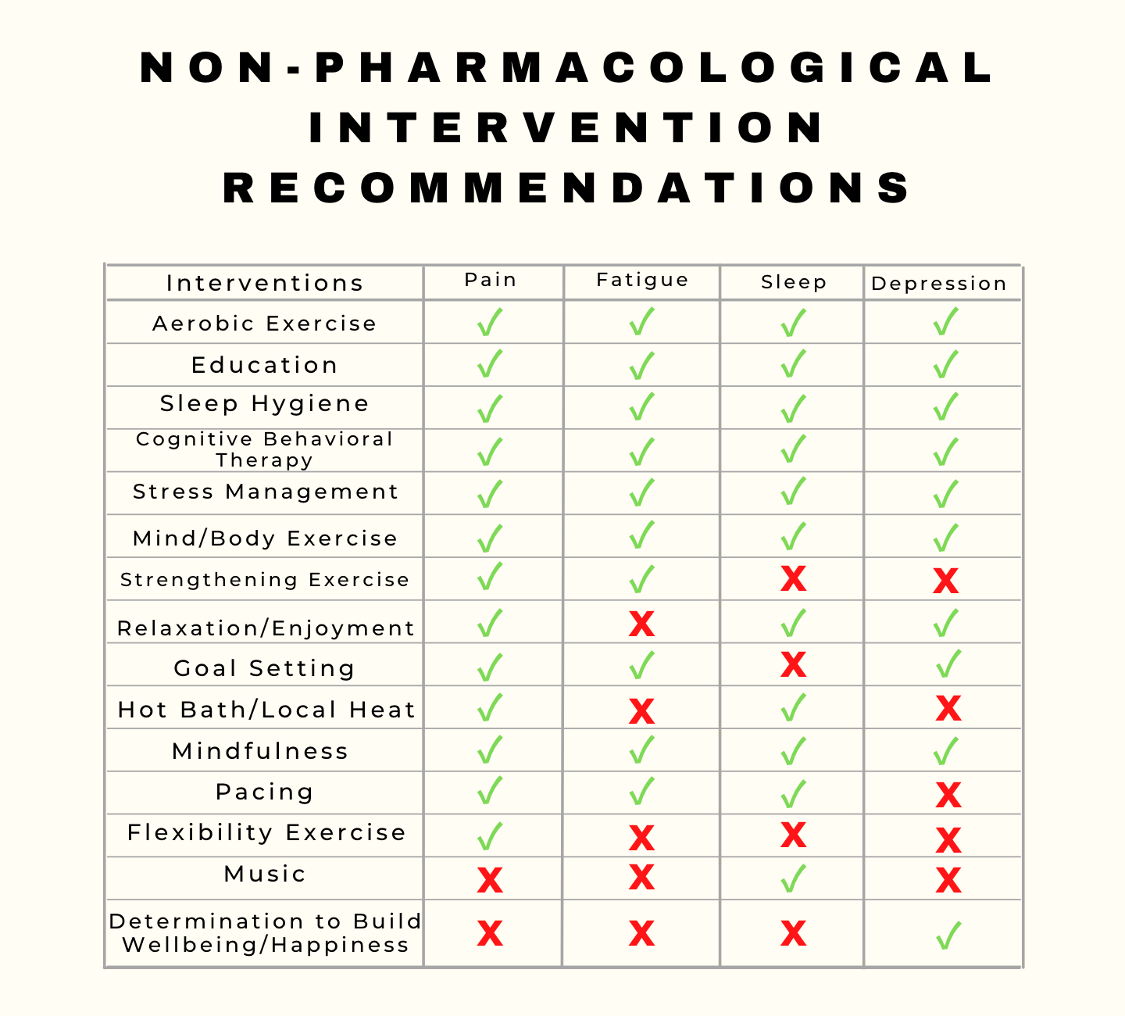
The Delphi exercise, which focused on non-pharmacological treatments for pain, fatigue, depression, and sleep problems in patients with fibromyalgia, provided evidence-based expert consensus recommendations that can be used to individualize treatments in clinical practice. Results identified aerobic exercise, cognitive behavioral therapy, sleep hygiene, and education as core interventions, according to a study published in Seminars in Arthritis and Rheumatism.1
“[Fibromyalgia] impacts on the patients’ health and quality of life (QoL), and on their significant others and presents a significant presents a large health economic burden,” investigators explained. “Non-pharmacological interventions are often recommended as first-line treatment for fibromyalgia. However, there are currently no recommendations on which non-pharmacological intervention(s) to offer for the initial management of the different symptoms associated with fibromyalgia, and, which of these to prioritize as core treatments.”
Authors of the Canadian Fibromyalgia Guidelines Group and the European Alliance of Associations for Rheumatology (EULAR) guidelines, as well as members of the American Pain Society and clinicians with fibromyalgia expertise were invited to participate in the 3-stage, international, multidisciplinary Delphi exercise. Participants chose non-pharmacological interventions that may be helpful in treating fibromyalgia symptoms, specifically sleep issues, depression, fatigue, and pain. They were then asked to further classify treatments as either core or adjunctive. To make the decision process easier, investigators provided an evidence summary. Items that obtained over 70% of votes were accepted, while those receiving less than 30% were rejected. Items between 30-70% of votes were recirculated until a consensus could be made. After consensus was achieved, 2 patients with fibromyalgia were able to review the results and analyze them from the patient perspective.
In total, 17 experts (Europe: n = 10; North America: n = 6; Israel: n = 1) participated in the exercise and completed all 3 rounds. Ultimately, the core treatments for all symptoms were education, sleep hygiene, aerobic exercise, and cognitive behavioral therapy. Regarding pain, fatigue, and sleep problems, mind-body exercises were recommended. Mindfulness was voted as a core treatment for depression and recommended as adjunctive treatment for other symptoms. Other adjunctive treatments included music, local heat, hot bath, and relaxation. The patient representatives agreed with the results of the expert consensus and added that the patient’s preferences and past experience should play a role in prescribing these treatments.
The study was strengthened by inviting a multidisciplinary, international group of clinicians and academics to participate, including some who have worked on developing fibromyalgia guidelines. Additionally, a comprehensive list of non-pharmacological interventions was presented, along with the option to suggest other interventions for each symptoms. However, the panel members were mostly physicians, which may have led to potential bias. Another limitation was that some interventions were grouped together, such as music intervention being defined as listening to any type of music for either pleasure or therapeutic purposes. Lastly, although depression was assessed, anxiety, a common symptom, was excluded from analysis.
“Personalizing treatment for fibromyalgia using a targeted symptom approach has been suggested as a way to improve outcomes for patients,” investigators concluded. “Clinicians may find this useful as an aid to shared decision-making and treatment choices with patients as part of an individualized management plan.”
Reference:
Kundakci B, Hall M, Atzeni F, et al. International, multidisciplinary Delphi consensus recommendations on non-pharmacological interventions for fibromyalgia [published online ahead of print, 2022 Sep 24]. Semin Arthritis Rheum. 2022;57:152101. doi:10.1016/j.semarthrit.2022.152101