The Latest in Testosterone Replacement Therapy
Partner Perspectives

The Latest in Testosterone Replacement Therapy (TRT): JATENZO® (testosterone undecanoate) Capsules CIII, an Oral Option for Patients with Hypogonadism
Introduction
There has been an explosion in treatment options for men with hypogonadism in the last 2 decades. The latest innovation in Testosterone Replacement Therapy (TRT) is JATENZO®, an oral softgel formulation of testosterone undecanoate (TU).1 Prior to the availability of JATENZO, treatment options in the United States (U.S.) included TRT injections, implantable pellets and several topical formulations.2,3 Even with so many dosing options available, many patients abandoned these kinds of treatments due to administration challenges (e.g., skin irritation for topicals, injection site pain for injectables).2,4
JATENZO is indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone: primary hypogonadism (congenital or acquired) and hypogonadotropic hypogonadism (congenital or acquired) due to structural or genetic etiologies.
What took so long to develop an oral TRT?
After the discovery of testosterone and its synthesis in the 1930s, it was quickly realized that oral testosterone does not have sufficient bioavailability to be effective as a replacement therapy due to rapid metabolism in the liver.3 A number of strategies have been explored to overcome this, including chemical modification of testosterone, which eventually led to the FDA-approval of 17 α-methyl-testosterone.3 However, long-term use of 17 α-methyl-testosterone, especially at higher doses, can cause potentially serious liver toxicity, so this compound is rarely used as a TRT currently.3
Testosterone esters were also developed, both as intramuscular injections (testosterone enthanate, cypionate, and TU)3,5 and for oral use (TU only).2,3 In fact, a formulation of oral TU has been widely available and used outside the U.S. since the 1970s.2,3 It was not approved for use in the U.S. because of its pharmacokinetic profile; it is often a struggle to maintain testosterone in the eugonadal range with this formulation.2 In contrast, JATENZO met the US FDA regulatory standards for pharmacokinetics, and safety and efficacy, and is the first oral TU to be approved for use in the U.S.1 It became available for prescription in February 2020.6
What is different about JATENZO?
The key innovation to the formulation is the self- emulsifying drug delivery system,2,7,8 which allows for the formation of TU-containing lipoprotein particles in the gut as the contents of the capsules disperse. (Figure) This allows TU to be absorbed via the intestinal lymphatics, bypassing the liver, and avoiding hepatic first-pass metabolism of testosterone.7,8
Figure
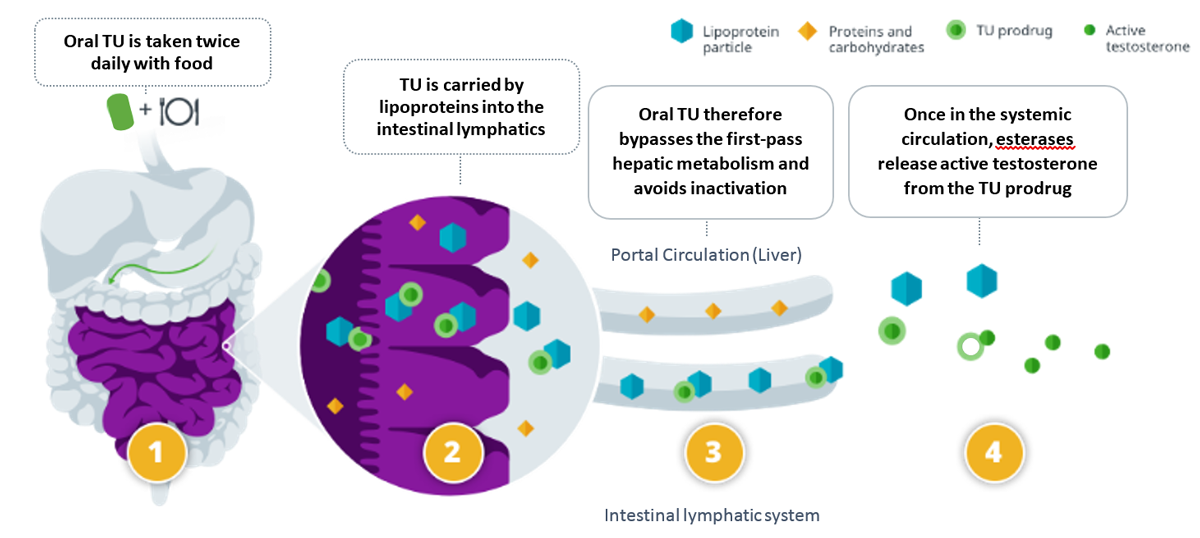
Once in the systemic circulation, TU is released from the lipoprotein particle, and endogenous esterases cleave active testosterone (T) from TU. The fatty acid (undecanoate) is then metabolized like dietary fat.8 (Figure) The selection of the undecanoate ester is important, as other fatty acids are less lipophilic and can be diverted to the liver instead of being taken up by the intestinal lymphatic system.2
The result is consistently sustained therapeutic levels; in clinical trials of JATENZO, Cmax was reached at about 2 hours after the morning dose, with the Cmin around 12 hours. The Cavg of serum T over 24 hours was 489 ± 158 ng/dL (mean ± SD) when expressed as approximate serum T equivalents based on assay of plasma T.1,9
The most common adverse events of JATENZO (incidence ≥2%) are headache (5%), increased hematocrit (5%), hypertension (4%), decreased HDL (3%), and nausea (2%). No clinically significant changes in liver function tests have been observed in clinical trials.1,8
How is the formulation dosed and titrated?
JATENZO capsules are available in 3 different strengths, allowing physicians to prescribe 5 dose levels to ensure each patient achieves serum testosterone in the eugonadal range.1
JATENZO is taken twice daily (BID) with food, once in the morning and once in the evening.1 The starting dose is 237 mg TU BID, and serum T should be measured to check for response to therapy after at least 7 days on treatment, about 6 hours after the morning dose is taken.1
With the addition of JATENZO to the treatment paradigm for hypogonadism, patients and physicians finally have an oral softgel option for this chronic condition. For more information, visit JATENZO.com/hcp/.
Peer-reviewed publications of Phase 3 studies of JATENZO have been published and are available in Therapeutic Advances in Urology and The Journal of Clinical Endocrinology & Metabolism.
Please see Important Safety Information, including BOXED WARNING on increases in blood pressure, below.
References:
- JATENZO® (testosterone undecanoate) [prescribing information]. Northbrook, IL: Clarus Therapeutics, Inc.; March 2019.
- Yin AY, et al. J Androl. 2012;33:190-201.
- Nieschlag E, Nieschlag S. Eur J Endocrinol. 2019;180:R201-R212.
- Schoenfeld MJ, et al. J Sex Med. 2013;10:1401-1409.
- FDA website. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=085635. Accessed April 30, 2020.
- Clarus Therapeutics announces commercial launch and availability of JATENZO (testosterone undecanoate) capsules, CIII for the treatment of hypogonadism [press release]. Northbrook, IL: GlobeNewswire; February 10, 2020.
- Gupta S, et al. ISRN Pharmaceutics. 2013 Dec 26;2013:848043. doi:10.1155/2013/848043.
- Data on file. Clinical Study Report: CLAR-15012. Clarus Therapeutics, Inc.
- Data on file. Clinical Study Report: CLAR-18019. Clarus Therapeutics, Inc.