Tocilizumab Treatment Associated with Cardiovascular Improvements in RA Patients
Though rheumatoid arthritis (RA) patients have a significantly higher risk of developing cardiovascular disease (CVD) compared to the general population, results from a pair of studies show that the biologic disease modifier Actemra (tocilizumab) may actually improve the cardiovascular (CV) profiles of RA patients.
Though rheumatoid arthritis (RA) patients have a significantly higher risk of developing cardiovascular disease (CVD) compared to the general population, results from a pair of studies presented at the American College of Rheumatology 2013 Annual Meeting in San Diego, CA, show that the biologic disease modifier Actemra (tocilizumab) may actually improve the cardiovascular (CV) profiles of RA patients.
As a follow-up to their previous open-label randomized controlled trial that found tocilizumab monotherapy reduces arterial stiffness in RA as effectively as etanercept or adalimumab monotherapy, Kensuke Kume, MD, of the Department of Rheumatology at the Hiroshima Clinic in Japan, and colleagues introduced new findings on the drug’s effects on left ventricular (LV) mass and cardiac output (CO) in RA patients during a poster session on small molecule and biologic RA therapies.
For that cohort study, the researchers administered tocilizumab in addition to methotrexate in 31 patients who had moderate-to-severe RA despite being treated with methotrexate prior to the study. The authors assessed LV morphology and function through cardiac MRI at baseline and 24 weeks, as well as CV risk factors, disease activity scores (DAS28), and C-reactive protein (CRP) levels at regular visits.
At the end of the study period, both left ventricular mass index (LVMI) and CO attenuated considerably with tocilizumab, and even right ventricular mass improved from baseline in 20% of the study participants. Furthermore, DAS28 and CRP improved significantly with the biologic therapy, although the authors noted that “tocilizumab improved LVMI and CO independently of its effects on disease activity.”
Recognizing that tocilizumab inhibits the Interleukin 6 (IL-6) receptor, the researchers concluded that “IL-6 might (play) an important role (in) LV hypertrophy,” which helps explain why tocilizumab “may prevent CV morbidity and mortality in RA.”
However, the authors of a second study proposed that the biologic’s effect on adiponectin levels can better illuminate the improved CV factors in RA patients who receive tocilizumab.
For their phase 3 MEASURE trial presented during the same poster session, Hoda Mirjafari, MBChB, of the Arthritis Research UK Epidemiology Unit at the University of Manchester, and researchers from the University of Glasgow, Columbia University, and Actemra manufacturer Genentech Inc. randomized moderate-to-severe RA patients with inadequate response to methotrexate to receive intravenous infusions of 8 mg/kg tocilizumab or placebo in combination with methotrexate every 4 weeks for about 6 months. At baseline and week 24, the investigators collected samples of fasting adiponectin — a protein hormone produced and secreted exclusively by fat cells that is thought to play an important role in atherosclerosis development — from 54 patients in the tocilizumab group and an equal number of patients in the placebo group.
Examining the baseline adiponectin samples between the two groups, the poster authors found that “older and female patients were more likely to have higher adiponectin levels” — which are linked to improved insulin sensitivity, lower triglyceride levels, and less atherosclerosis — whereas “physician diagnosis of diabetes and higher body mass index (BMI) were significantly associated with lower adiponectin levels.” Nevertheless, baseline physical function and DAS28 and CRP scores had no significant relation to adiponectin levels.
Comparing week 24 data from both participant groups, the researchers discovered that “treatment with tocilizumab 8 mg/kg plus methotrexate — but not with placebo — led to significant increases from baseline in adiponectin and lipid levels, and in significant decreases in inflammatory and disease activity parameters” (Table).
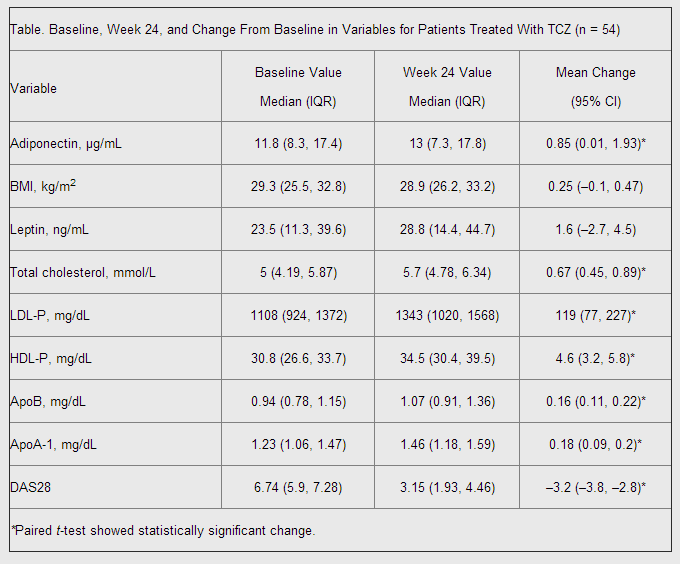
Thus, Mirjafari and her co-authors concluded that “adiponectin is increased with tocilizumab treatment in RA patients, with higher increases observed in patients with greater degrees of RA disease activity suppression.” However, the authors noted that future studies are still needed in order to “confirm whether tocilizumab increases adiponectin level and, if so, whether adiponectin change predicts CVD risk change with tocilizumab.”