Triglycerides and EPA: A New Chapter in CV Disease Prevention
Findings from REDUCE-IT are the first to show that EPA lowers the relative risk of hard cardiovascular endpoints. Johns Hopkins' Seth Martin, MD, and colleagues put the trial in perspective.
©Kerdkanno/Shutterstock.com

Figure. (Please click to enlarge)
Results of REDUCE-IT (Reduction of Cardiovascular Events With EPA - Intervention Trial), presented last week at the American Heart Association (AHA) Scientific Sessions 2018 have succeeded where findings from multiple trials of omega-3 fatty acids (OM3FA) have failed for years: The results offer hope.
Deepak Bhatt, MD, and colleagues reported that treatment with 4 mg daily of icosapent ethyl resulted in a 25% lower relative risk of the primary efficacy endpoint-a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina-vs placebo. The results have been welcomed, this time, with more optimism than caution; they have been a long time coming.
Beginnings
In 1978 Dyerberg and Bang reported high plasma levels of OM3FA were associated with a low incidence of myocardial infarction (MI) in Greenland Eskimo populations.1 Naturally occurring sources of OM3FAs, primarily eicosapentaenoic acid (EPA) and docosoahexaenoic acid (DHA), are salmon, tuna, and walnuts. Current OTC OM3FA, or “fish oil” supplements offer a range of combined amounts of EPA (300-700mg) and DHA (200-500mg), in addition to other fats and unlabeled OM3FAs. Unfortunately, these low-dose supplements may do little more than give patients a fishy after taste and an upset stomach.
There are currently four prescription-strength OM3FAs with FDA approval for treatment of hypertriglyceridemia ≥500 mg/dL, three of which contain combined EPA/DHA and one that contains EPA only:
- Epanova (polyunsaturated fatty acids 850 mg, ~75% combined EPA/DHA per 1 g capsule)
- Lovaza (EPA 465 mg, DHA 375 mg per 1 g capsule)
- Omtryg (EPA 465 mg, DHA 375 mg per 1.2 g capsule)
- Vascepa (EPA [icosapent ethyl], ~1 g per 1 g capsule)
OM3FA combinations for CVD disappoint
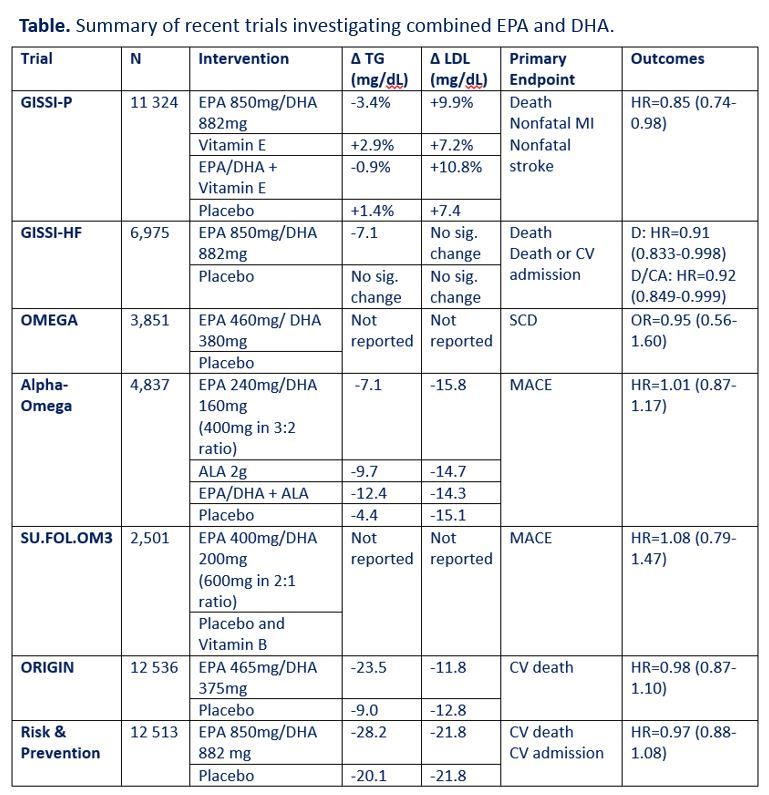
In the 4 decades since the report of Dyerberg and Bang, thousands of articles investigating the connection between OM3FAs and cardiovascular disease have been indexed in PubMed. In recent years, large-scale randomized controlled trials have investigated cardiovascular outcomes with supplementation of combined EPA and DHA-and results have been largely disappointing (see Table above). In these trials, doses of EPA have ranged from 240-850 mg and doses of DHA have ranged from 160-882 mg. The GISSI trials showed improvement in cardiovascular outcomes in patients taking combined EPA/DHA; however, subsequent trials investigating EPA/DHA have been negative.2-8
OM3FA monotherapy
A 2011 meta-analysis investigated the differential effects of EPA and DHA as monotherapy. Data from seventeen randomized placebo-controlled trials using DHA alone showed an increase in LDL cholesterol (LDL-C) by 7 mg/dL (95% CI, 3.98-10.5). No significant change was seen in LDL-C in the 10 trials investigating EPA alone.9 The mechanism by which DHA raises LDL-C is unclear; nevertheless, the rise in LDL-C may account for failure of combined OM3FA products to improve cardiovascular outcomes. It is notable that many of these trials have used LDL-C levels calculated by the Friedewald equation, which has been shown to underestimate LDL-C levels when triglycerides are ≥150 mg/dL.10 Thus, it is likely that initial calculated LDL-C values were artificially low and then as triglycerides were lowered this phenomenon was less at play, which would lead to an artifactual increase in LDL-C.
In 2012, the FDA approved the EPA icosapent ethyl (Vascepa) to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia.11 As noted, this fourth prescription-strength fatty acid is composed of only EPA (EPA ~1 g per 1 g capsule). The comparative efficacy for EPA vs OMF3FA combination therapies is not clear since there have not been head-to-head trials and across trial comparison are complicated by differences in participant characteristics. The proposed mechanism by which EPA, and other OM3FAs, reduce triglycerides is induction of beta oxidation of free fatty acids and inhibition of acyl-CoA:1,2-diacylglycerol acyltransferase, the enzyme that combines diacylglycerol with a single free fatty acid in synthesis of triglycerides. However, there may be other mechanisms of action as well, and ultimately we are most interested in their net effect on patient outcomes including cardiovascular disease.
From TGs to CVD
Cardiovascular disease related death has steadily declined since the 1980s.12 Much of this decline can be attributed to the introduction of statins. However, despite optimization of statin therapy and adequate reduction of LDL-C, substantial residual cardiovascular risk remains.13 Recent and ongoing trials show that EPA therapy and triglyceride level reduction is a promising means of mitigating this residual risk.14-17
The JELIS trial (2007) studied 18 645 individuals with a median triglyceride level of 153 mg/dL (IQR 109-220 mg/dL) from sites throughout Japan, who were randomized, in open-label fashion to receive icosapent ethyl (1.8 g daily) + statin or statin only. The primary endpoint was a composite of sudden cardiac death, fatal and non-fatal myocardial infarction, or other non-fatal cardiac events (unstable angina or coronary artery revascularization). This study population was followed for a mean of 4.6 years. The icosapent ethyl + statin group had a 5% (p<.0001) decrease in triglyceride levels compared to the control group. There was no significant difference in LDL-C reduction between groups. There was a 19% relative risk reduction in major coronary events (p=0.011).16 Critiques of the trial focused on higher baseline serum EPA levels and lower cardiovascular risk among the Japanese, compared to western populations. In a more recent (2016) study icosapent ethyl in higher doses (4 g daily) was shown to raise the serum levels of EPA in western populations to levels comparable to those in the Japanese-only population in the JELIS trial.18
The US experience
In the United States, two small trials have investigated lipid changes with high-dose (4g) icosapent ethyl. The MARINE trial (2011)14 studied 229 participants with very high triglyceride levels (500-2000 mg/dL; mean 679.5 mg/dL), who were randomized to receive icosapent ethyl 4 g daily or 2 g daily. In the group receiving 4 g daily triglycerides were reduced by 33% (p<.0001) vs 20% (p<.0051) in the group receiving 2 g daily. Adverse events were low and did not differ between groups.14 Similarly, the ANCHOR trial (2012) investigated 702 patients with high triglyceride levels (200-500 mg/dL) receiving icosapent ethyl 4 g daily or 2 g daily. Triglycerides decreased by 22% (p<.0001) and 10% (p<.001) in the 4 g daily group and 2 g daily group, respectively.15
Further analysis of the MARINE and ANCHOR study populations showed a significant reduction in remnant-like particle cholesterol (RLP-C) levels in patients receiving icosapent ethyl 4 g daily (MARINE -29.8%, p=0.004; ANCHOR -25.8%, p=0.0001).19 Though not powered for cardiovascular outcomes, these studies showed the ability of icosapent ethyl to significantly reduce triglyceride and RLP-C levels, both of which contribute to progression of atherosclerotic disease. These results raised the question of the ability of icosapent ethyl to improve cardiovascular outcomes.
REDUCE-IT
The stage was now set for the REDUCE-IT trial (2018), findings of which were highlighted at AHA Scientific Sessions 2018 and described in brief above.
The REDUCE-IT study population consisted of an international population of 8,179 patients with cardiovascular disease or risk factors for cardiovascular disease, controlled LDL-C, and triglyceride levels of 135-499 mg/dL; they received either 2 g of icosapent ethyl twice daily (4 g daily) or placebo. The composite endpoint of cardiovascular death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina was reached in 17% in the icosapent ethyl group compared to 22% in the placebo group (HR=0.75; 95% CI 0.68-0.83; p=0.00000001). Cardiovascular death was significantly less in the icosapent ethyl group (4.3% vs. 5.2%; HR=0.80; 95% CI 0.66-0.98; p=0.03).20
More data, soon
Further study of the benefits of icosapent ethyl is ongoing in other clinical trials. The EVAPORATE trial will offer further insight into the effect of icosapent ethyl on atherosclerosis as assessed by CT angiography. The aim is to determine the effect of 4 g daily therapy on changes in size and composition of coronary atherosclerotic plaques. Approximately 90 statin-treated patients in North America with coronary atherosclerosis, triglyceride levels of 200-499 mg/dL, and LDL-C levels of 40-115 mg/dL have been enrolled. The primary endpoint is change in the CT angiography measurement of low-attenuation coronary atherosclerotic plaque volume.17 The study is anticipated to conclude in late 2019; results will provide radiographic evidence of the effect of icosapent ethyl on atherosclerotic plaques, providing insight into the mechanisms underlying the positive outcomes of the REDUCE-IT trial.
The history of OM3FA investigation has consisted until just recently of disappointment generated by negative trial after negative trial. But now, forty years after the original report associating OM3FAs and decreased incidence of MI, OM3FAs have made an exciting comeback to the preventive cardiology field. In addition to the proven ability of EPA to reduce triglyceride levels, investigation will now continue to elucidate the effects of EPA leading to reduction in cardiovascular mortality.
References:
1. Dyerberg J, Bang HO. Dietary fat and thrombosis. In: Lancet. Vol 1. England1978:152.
2. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447-455.
3. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223-1230.
4. Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152-2159.
5. Kromhout D, Giltay EJ, Geleijnse JM, Group AOT. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015-2026.
6. Galan P, Kesse-Guyot E, Czernichow S, et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273.
7. Bosch J, Gerstein HC, Dagenais GR, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309-318.
8. Roncaglioni MC, Tombesi M, Avanzini F, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800-1808.
9. Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13(6):474-483.
10. Martin SS, Blaha MJ, Elshazly MB, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013;62(8):732-739.
11. Vascepa [package insert]. In. Bedminster, NJ: Amarin Pharma, Inc; 2012.
12. Prevention CfDCa. QuickStats: Age-Adjusted Death Rates* for Heart Disease and Cancer, † by Sex - United States, 1980–2011. Morbidity and Mortality Weekly Report (MMWR) Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6337a6.htm. Published 2014. Accessed.
13. Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet Need for Adjunctive Dyslipidemia Therapy in Hypertriglyceridemia Management. J Am Coll Cardiol. 2018;72(3):330-343.
14. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108(5):682-690.
15. Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110(7):984-992.
16. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090-1098.
17. Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: Rationale and design of the EVAPORATE study. Clin Cardiol. 2018;41(1):13-19.
18. Bays HE, Ballantyne CM, Doyle RT, Juliano RA, Philip S. Icosapent ethyl: Eicosapentaenoic acid concentration and triglyceride-lowering effects across clinical studies. Prostaglandins Other Lipid Mediat. 2016;125:57-64.
19. Ballantyne CM, Bays HE, Philip S, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): Effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis. 2016;253:81-87.
20. Deepak L. Bhatt MD, M.P.H., P. Gabriel Steg MD, Michael Miller MD, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. New England Journal of Medicine. 2018.