JAK2 Inhibitor Improves Quality of Life for Patients with Myelofibrosis
A phase I/II study presented at the 51st Annual Meeting of the American Society of Hematology in New Orleans, Louisiana, found that INCB018424, an experimental JAK2 inhibitor, demonstrated activity in patients with myelofibrosis. INCB018424 shrank enlarged spleens, helped patients gain weight, reduced pain, and increased patients’ capacity to exercise, all of which significantly improved their quality of life.
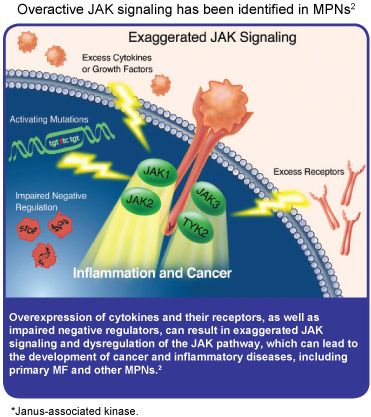
neoplasms, with m
NEW ORLEANS, LA — Approximately 3000 new cases of myelofibrosis (also called agnogenic myeloid metaplasia or idiopathic myelofibrosis) are diagnosed annually in the United States. This bone marrow disorder is the most serious of chronic myeloproliferative ost patients experiencing massive swelling of the spleen, weight loss, fatigue, abdominal discomfort, bone and muscle pain, and night sweats. Some patients also develop severe itching.
Several genetic mutations are associated with myelofibrosis, but the JAK2V617F mutation appears to be the most prevalent, occurring in approximately 50% of patients. This mutation leaves the Janus-associated kinase 2 (JAK2) enzyme permanently turned on, which results in an overgrowth of bone marrow cells and eventually leads to myelofibrosis. There are no treatments approved specifically for myelofibrosis and researchers have previously not been able to target the abnormal signaling pathway in the malignant cells that contributes to this disease. A phase I/II study presented at the 51st Annual Meeting of the American Society of Hematology in New Orleans, Louisiana, however, found that INCB018424, an experimental JAK2 inhibitor, demonstrated activity in patients with myelofibrosis. INCB018424 shrank enlarged spleens, helped patients gain weight, reduced pain, and increased patients’ capacity to exercise, all of which significantly improved their quality of life.
The study included 155 patients with primary myelofibrosis, post-polycythemia vera myelofibrosis, or post-essential thrombocythemia myelofibrosis. Patients were treated with INCB018424 for a median of at least 1 year (n = 76).Clinical responses were maintained over the entire duration of treatment, with 74% of patients (n = 115) remaining on therapy at the time the study results were reported. Three patients had progression to acute myelogenous leukemia, which is fewer than expected based on previously published data (
).
Patients started with a 10 mg or 15 mg dose of INCB018424 twice daily; after 1 to 2 months of therapy, dose titration was permitted. At the optimal dose of 15 mg to 20 mg twice daily, reduction of spleen volume was evident after 1 month and persisted over 6 months of therapy. Nearly half (48%) of patients experienced shrinkage of 35% or higher. Investigators observed a rapid and lasting improvement in symptom score, with 51% of patients achieving a 50% reduction at 1 month and 58% of these patients maintaining that reduction for at least 6 months. In addition, treatment with INCB018424 allowed patients to increase the distance of their 6-minute walk by a median of 33 meters at 1 month and 70 meters at 6 months. Because INCB018424 inhibits JAK2 in healthy cells, it can result in low blood counts. This was dose-limiting for some patients.
An important and unexpected finding of the study was that even patients without JAK2 mutations responded to the novel drug. According to Srdan Verstovsek, MD, PhD, associate professor, department of leukemia, MD Anderson Cancer Center, Houston, Texas, who presented these findings, the activity of INB018424 in patients without the mutation suggests that these patients “still have a very active JAK signaling pathway and can benefit from JAK inhibition.” Pivotal registrational studies for INB018424 in myelofibrosis are currently underway. Visit
for more information on these trials. ASH Abstract 756.
Disclosure: This phase I/II clinical trial was funded by Incyte Corporation, and Dr Verstovsek has received funding from Incyte.