Assessing atherosclerosis in rheumatologic disease
In the past decade, evidence increasingly has suggested a higher prevalence of atherosclerotic cardiovascular disease (CVD) in patients with autoimmune rheumatologic conditions.
In the past decade, evidence increasingly has suggested a higher prevalence of atherosclerotic cardiovascular disease (CVD) in patients with autoimmune rheumatologic conditions. Because the role of inflammation in atherogenesis has been appreciated, a positive association of CVD with chronic inflammatory diseases is not surprising. However, the pathogenesis of atherosclerosis in these patients is complex and appears to involve both traditional and disease-related CVD risk factors.
Physicians who are managing patients with rheumatologic disease should be aware of this increase in CVD risk. Medical management of the rheumatologic disease with drugs associated with lower risk of CVD events ultimately may minimize morbidity and mortality related to atherosclerosis. Patients also should be counseled about the benefits of altering modifiable risk factors, such as cigarette smoking, diet, and a sedentary lifestyle.
In this article, we examine the evidence that links CVD with 3 common rheumatologic diseases: rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and spondyloarthropathy (SpA). Understanding these connections may provide insights that can help the physician reduce the patient's cardiac risk and successfully address the special challenges created by concomitant CVD and rheumatologic disease.
ASSOCIATED RHEUMATOLOGIC DISEASES
Rheumatoid arthritis
RA is a chronic, systemic autoimmune disease that affects about 1.3 million Americans.1 Life expectancy is reduced by 7 to 23 years. CVD is the leading cause of death in patients with RA, accounting for nearly 40% of deaths.2
Numerous reports consistently have shown an increase in the risk of myocardial infarction (MI) in patients who have RA compared with persons who do not. In a study of 236 patients with RA compared with 4635 non-RA participants in a population-based cohort, the incidence rate ratio for CVD events (MI and revascularization procedures) was 3.96 (95% confidence interval [CI], 1.86-8.43) after adjusting for traditional CVD risk factors.3
In the Nurse's Health Study, which compared CVD event rates among the 527 women in whom RA had developed with the CVD event rates among the non-RA participants, the adjusted relative risk (RR) for MI was 2.0 (95% CI, 1.23-3.29). For those women who had had RA for 10 years or more, the adjusted RR of MI was 3.10 (95% CI, 1.64-5.87).4
In another prospective cohort study, 17,738 patients with RA were compared with 3001 patients who had noninflammatory rheumatologic disorders. The hazard ratio of first MI was 1.9 (95% CI, 1.2-2.9, P = .005).5
Evidence also suggests an increase in risk of cerebrovascular accident, or stroke, in patients with RA. In a nested case-control study within a longitudinal databank, 269 patients with first stroke were matched with up to 20 controls for each case. Of the 67 ischemic strokes, 41 were in patients with RA. The odds ratio for the risk in all-category stroke and in ischemic stroke in RA was 1.64 (95% CI, 1.16-2.30, P = .005) and 2.66 (95% CI 1.24-5.70, P = .012), respectively.6 Although the risk of stroke was found to be increased in the participants with RA in the Nurse's Health Study, this increased risk was not statistically significant.4
All together, these studies show an increase in risk of CV events related to multiple arterial beds in patients with RA. The increase in risk may be 2- to 3-fold, depending on RA duration and subjects studied.
The positive association of RA with CV events is independent of traditional risk factors for CVD, although traditional risk factors are common in these patients. For example, patients with RA are more likely to be cigarette smokers and have hypertension than are patients with osteoarthritis.7 Although the prevalence of diabetes mellitus (DM) in subjects with RA is not greater than in those without RA,7,8 a positive association between insulin resistance and subclinical coronary atherosclerosis has been reported.9 The relationship between RA and dyslipidemia is a complex one; evidence suggests that newly diagnosed RA is associated with an adverse lipid profile that may improve after management of joint disease.10
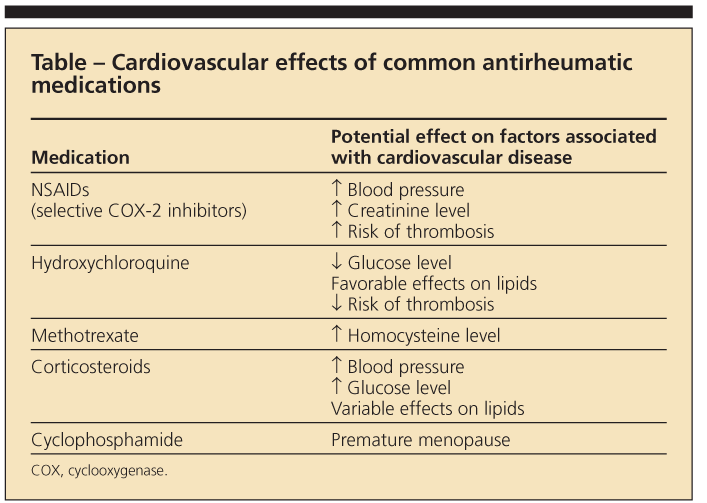
It has been suggested that RA-related risk factors-the chronic inflammatory milieu and, possibly, adverse effects of RA medications (Table)-confer the additional risk of CVD.11 For example, factors associated with increased CVD risk in patients who have RA include longer disease duration, higher levels of disease activity and severity, and the presence of the HLA-DRB1*0404 allele.12
There are some common features in the pathophysiology of atherosclerosis and RA. In atherosclerosis, there is vascular inflammation of the vessel wall as manifested by mononuclear cell infiltration, elevated levels of cytokines, increased cellular adhesion, and plaque destabilization.13 RA is characterized by inflammation in the synovium as well as elevated serum levels of C-reactive protein (CRP), tumor necrosis factor a (TNF)-α, interleukin (IL)-1, and IL-6. These inflammatory mediators also may directly promote vascular disease, and TNF-α and IL-6 probably contribute to insulin resistance described in RA.9,14
Use of corticosteroids and selective cyclooxygenase 2 (COX-2) inhibitors has been associated with a higher risk of CV events.15,16 The effect of corticosteroids on individual CV risk factors is mixed. Although these agents may contribute to risk by causing hypertension, DM, dyslipidemia, and truncal obesity, low-dose corticosteroids also may improve disease activity, thus potentially reducing systemic inflammation. Their association with CV events in RA is most striking when they are used at higher daily doses and with greater cumulative exposure.15
Use of nonselective NSAIDs may result in a rise in blood pressure and creatinine levels. In vitro studies suggest that ibuprofen used with low-dose aspirin for CV protection may negate the cardioprotective antiplatelet effects of aspirin because of competitive binding of COX-1.17
Other RA medications have not been associated with altered risk of CVD on a consistent basis. Use of TNF-α inhibitors has been associated with worsening congestive heart failure (CHF) but not with CVD.11,12
Methotrexate use has been associated with a nearly 70% reduction in CVD mortality, although this result has not been consistent across all studies.11,18 Hydroxychloroquine is associated with a reduced risk of incident DM in patients with RA and has antiplatelet and lipid-lowering effects, although its relationship with CVD is unclear.19,20
Recent interest in the dual anti-inflammatory and lipid-lowering effects of hydroxymethylglutaryl-coenzyme A (HMG-CoA) inhibitors or statins has led to trials of these drugs in the treatment of inflammatory and autoimmune diseases.21,22 In a randomized double-blind trial, 116 patients with active RA received 40 mg of atorvastatin or placebo as an adjunct to existing antirheumatic therapy.23 At 6 months, clinical disease activity as measured by the Disease Activity Score 28 (DAS28) improved, and the CRP level, erythrocyte sedimentation rate, and swollen joint count decreased significantly in the patients who were receiving atorvastatin. However, the overall effect was modest.
Based on the limited data available currently, the use of statins in patients with RA who do not meet the criteria for treatment of dyslipidemia is not routinely recommended. Furthermore, management of dyslipidemia is ideally based on laboratory testing during quiescent joint disease, when active systemic inflammation is less likely to have an impact on screening results.
Patients who have RA may be more likely to experience unrecognized MI and sudden cardiac death and less likely to report symptoms of angina than patients who do not have RA.24 Although the reason for occult clinical CVD is unclear, this observation underscores the importance of physicians closely monitoring patients with RA for the development of CVD symptoms. Other causes of chest pain in patients with RA include serositis, gastroesophageal reflux, esophagitis, and NSAID-related gastropathy. However, chest pain in patients with RA should alert the physician to the presence of ischemic heart disease while he or she considers other etiologies.
Systemic lupus erythematosus
This chronic, pleomorphic, systemic autoimmune disease affects about 161,000 to 322,000 Americans1; it has a female to male predominance of 9 to 1. In most women, the disease develops during their reproductive years.
In 1976, Urowitz and colleagues25 reported a bimodal mortality pattern in patients with lupus: early deaths were related to active lupus or treatment-related complications, such as infection, and late deaths were related to CVD. A study from the Johns Hopkins Lupus Cohort found the prevalence of CVD, defined as angina or MI, to be 8.3%.26 Similar results were reported from a prospective cohort of 1087 patients with SLE monitored for 34 years; CVD prevalence was 10.9% in these patients and 9.6% in the inception cohort of 561 patients.27
In another study, 498 women with SLE were compared with 2208 women of similar age in the Framingham Offspring Study. Those with SLE in the 35- to 44-year-old group were more than 50 times more likely to have an MI (rate ratio, 52.43; 95% CI, 21.6-98.5).28 Factors more common in women with SLE who had a CVD event were older age at SLE diagnosis, longer disease duration, longer duration of corticosteroid use, hypercholesterolemia, and postmenopausal status.
This increased risk appears to be independent of traditional CVD risk factors29,30 that are more prevalent in patients with SLE,26,31 suggesting a relationship between CVD and the disease itself or its treatments (see Table). The pathogenesis of SLE involves immune complex formation, endothelial deposition of these circulating immune complexes, and direct vascular damage. Factors that may play a role in accelerated atherogenesis in SLE include age at diagnosis, longer lupus duration, and higher serum homocysteine levels.32
The differential diagnosis of chest pain or dyspnea on exertion in patients with SLE is quite broad. It can be the result of pleuritis or pericarditis, myocarditis, valvular disease, pulmonary embolus, pulmonary hypertension, pneumonia, or gastroesophageal reflux with esophagitis related to the use of NSAIDs. However, CVD always should be considered in the diagnostic evaluation.
Spondyloarthropathies
Although not studied as extensively as RA and SLE, the SpAs also appear to be associated with an increased risk of CVD. Han and associates33 reported an increased prevalence of CVD in RA, psoriatic arthritis, and ankylosing spondylitis. The prevalence ratios of ischemic heart disease relative to controls in the 3 disease groups were 1.5, 1.3, and 1.2, respectively, and those of all CVD (including peripheral vascular disease and CHF) were 1.6, 1.3, and 1.7, respectively.
A large prospective cohort study of patients with psoriasis noted that this condition is an independent risk factor for MI. The study included patients with mild (n = 127,139) and severe psoriasis (n = 3837) compared with 556,995 controls. The RR of MI was greatest in younger patients who had severe psoriasis. For example, a 30-year-old patient with severe psoriasis was determined to have an RR of MI of 3.10 (95% CI, 1.98-4.86) compared with the control group.34 Part of this increased risk is conferred by the metabolic syndrome, a condition associated with CVD risk that may be more prevalent in this patient population.35
DETECTION OF SUBCLINICAL CVD
When physicians take care of patients who have a rheumatologic disease, particularly RA or SLE, a high index of suspicion for CVD is required, even in the "unlikely suspects" (patients not typically considered to be at high risk for CVD with traditional risk factor assessment). Physicians should be prompt and aggressive in evaluating patients for ischemic heart disease or stroke in the presence of suggestive symptoms, recognizing that these symptoms may be atypical or underreported.
Noninvasive tests have been used to measure preclinical atherosclerosis in patients with rheumatologic disease.32,36-38 This approach allows for detection of early vascular changes in atherogenesis-such as arterial stiffness, an increase in arterial intima-media thickness (IMT), plaque, and calcification in arterial plaque-before clinical events occur.
Arterial stiffness is assessed using pulse-wave velocity and flow-mediated dilatation of the brachial artery induced by a vasodilator. Ultrasonography of the carotid arteries is used to measure IMT and plaque. Electron beam CT detects the presence of arterial calcification and generates a quantified estimate of calcified plaque with a calcium score in the major coronary arteries and aorta.
These studies are important research tools. However, none of these tests for subclinical atherosclerotic vascular disease is currently recommended for screening in daily practice. Because "positive" findings of subclinical disease have uncertain positive predictive value in patients with rheumatologic diseases, the use of these tests for clinical management in asymptomatic patients currently is unsubstantiated by published data.
RECOMMENDATIONS
Primary prevention of CVD is of paramount importance. Physicians should reinforce lifestyle modification measures, such as smoking cessation, regular physical activity, maintaining a healthy diet, weight management, and stress reduction strategies. DM, hypertension, and hyperlipidemia should be controlled. Because active inflammatory disease may impact metabolic risk factors for CVD, screening for these tests ideally is done when joint disease is optimally managed.
Although both RA and SLE arguably confer CV risk equivalent to that of DM, no studies to date have indicated better CVD outcomes with risk factor reduction at targets set for high-risk participants rather than the general population. Patients and primary care physicians should be informed of the increased risk of CVD to optimize the chances of risk factor reduction and prompt recognition of clinical CVD.
Although low-dose aspirin has been suggested for primary CV prophylaxis, this approach is controversial in patients who have RA or SLE. The benefits of daily aspirin may be offset by an increased risk of GI bleeding.
If the patient and the physician find aspirin use for CV prophylaxis desirable, and the patient is using concomitant nonselective NSAIDs, sequential dosing of the agents is a reasonable option. Aspirin taken 2 hours before the first daily NSAID dose may optimize aspirin's cardioprotective antiplatelet effects.11 However, the combination of aspirin with NSAIDs increases the risk of GI bleeding and, if the patient and the physician make the decision to use these drugs concomitantly, then a proton pump inhibitor or misoprostol should be added to the regimen.16 The risks versus the benefits of NSAIDs and COX-2 inhibitors include not only their effects on CV risk factors (eg, blood pressure, renal function, and hemostasis) but also their beneficial anti-inflammatory and corticosteroid-sparing effects.
While minimizing the use of drugs associated with higher CVD risk, optimal control of the underlying rheumatologic disease appears to reduce CV morbidity and mortality.11,18 Thus, corticosteroids should be minimized and alternative therapy (eg, methotrexate and hydroxychloroquine) should be initiated to reduce daily and cumulative corticosteroid exposure.19,20 In patients with RA, TNF-α inhibitors appear to be reasonable corticosteroid-sparing agents in the absence of CHF, although other treatment-related risks, particularly serious infections, also must be considered.11,12
CONCLUSIONS
Physicians should be aware of the increase in CV risk when managing patients who have a rheumatologic disease. Patients should be counseled about altering modifiable risk factors. Medical management of the rheumatologic disease with drugs associated with lower risk of CV events may minimize morbidity and mortality related to atherosclerosis.
References:
References1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part 1. Arthritis Rheum. 2008;58:15-25.
2. Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481-494.
3. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
4. Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303-1307.
5. Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis. Arthritis Rheum. 2008;58:2612-2621.
6. Nadareishvili Z, Michaud K, Hallenbeck JM, Wolfe F. Cardiovascular, rheumatologic, and pharmacologic predictors of stroke in patients with rheumatoid arthritis: a nested, case-control study. Arthritis Rheum. 2008;59:1090-1096.
7. Gabriel SE, Crowson CS, O'Fallon WM. Comorbidity in arthritis. J Rheumatol. 1999;26:2475-2479.
8. del Rincon I, Freeman GL, Haas RW, et al. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413-3423.
9. Chung CP, Oeser A, Solus JF, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58:2105-2112.
10. Park YB, Choi HK, Kim, MY, et al. Effects of antirheumatic therapy on serum lipid levels in patients with rheumatoid arthritis: a prospective study. Am J Med. 2002;113:188-193.
11. Pham T, Gossec L, Constantin A, et al. Cardiovascular risk and rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine. 2006;73:379-387.
12. Wasko MC. Rheumatoid arthritis and cardiovascular disease. Curr Rheumatol Rep. 2008;10:390-397.
13. Quyyumi AA. Inflamed joints and stiff arteries: is rheumatoid arthritis a cardiovascular risk factor? Circulation. 2006;114:1137-1139.
14. Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how "high-grade" systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957-2963.
15. Davis JM 3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;56:820-830.
16. American College of Rheumatology Ad Hoc Group on Use of Selective and Nonselective Nonsteroidal Antiinflammatory Drugs. Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59:1058-1073.
17. Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809-1817.
18. Choi HK, Hernan MA, Seeger JD, et al. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173-1177.
19. Petri M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus. 1996;5(suppl 1):S16-S22.
20. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
21. Ridker PM, Cannon CP, Morrow D, at al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
22. Abeles AM, Pillinger MH. Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy? Arthritis Rheum. 2006;54:393-407.
23. McCarey DW, McInnes IB, Madhok R, at al. Trial of atorvastatin in rheumatoid arthritis (TARA): double-blind, randomised, placebo-controlled trial. Lancet. 2004;363:2015-2021.
24. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402-411.
25. Urowitz MB, Bookman AA, Koehler BE, et al. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221-225.
26. Petri M, Spence D, Bone LR, Hochberg MC. Coronary artery disease risk factors in the Johns Hopkins Lupus Cohort: prevalence, recognition by patients, and preventive practices. Medicine (Baltimore). 1992;71:291-302.
27. Urowitz MB, Ibanez D, Gladman DD. Atherosclerotic vascular events in a single large lupus cohort: prevalence and risk factors. J Rheumatol. 2007;34:70-75.
28. Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408-415.
29. Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331-2337.
30. Lee AB, Godfrey T, Rowley KG, et al. Traditional risk factor assessment does not capture the extent of cardiovascular risk in systemic lupus erythematosus. Intern Med J. 2006;36:237-243.
31. Bruce IN, Urowitz MB, Gladman DD, et al. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48:3159-3167.
32. Roman MJ, Crow MK, Lockshin MD, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3412-3419.
33. Han C, Robinson DW Jr, Hackett MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167-2172.
34. Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
35. Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
36. Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407-2415.
37. Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399-2406.
38. Roman MJ, Moeller E, Davis A, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144:249-256.