Publication
Article
Evaluating the Evidence: Direct Comparative Trials in Multiple Sclerosis
Author(s):
Although 10 disease-modifying agents are available for patients with relapsing-remitting multiple sclerosis (RRMS), and more than 20 years have elapsed since the approval in the United States of the first traditional injectable agents for RRMS, until 2012 only 6 large head-to-head trials had evaluated these treatments directly.
Although 10 disease-modifying agents are available for patients with relapsing-remitting multiple sclerosis (RRMS), and more than 20 years have elapsed since the approval in the United States of the first traditional injectable agents for RRMS, until 2012 only 6 large head-to-head trials had evaluated these treatments directly.1
With the new oral agents, direct comparative data are becoming more plentiful. Two clinical trials -- CONFIRM (with oral dimethyl fumarate [DMF]) and TRANSFORMS (with oral fingolimod) -- compared these 2 oral agents with traditional injectable agents.2-3
Comparative Data with Traditional Injectable Agents
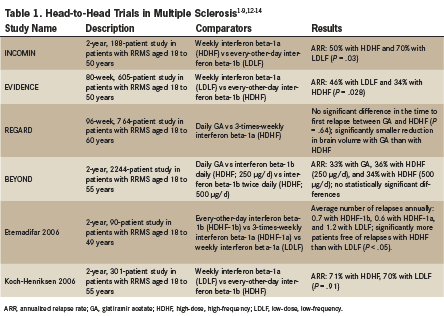
In 6 comparative trials (INCOMIN, EVIDENCE, REGARD, BEYOND, Etemadifar 2006, and Koch-Henriksen 2006) investigators evaluated 3 classes of traditional agents: high-dose, high-frequency interferons; low-dose, low-frequency interferons; and glatiramer acetate (Table 1).4-8
The INCOMIN, EVIDENCE, and Etemadifar 2006 studies demonstrated lower relapse rates with high-dose, high-frequency interferons than with low-dose, low-frequency interferons. Although these results were not confirmed by the Koch-Henriksen 2006 study, a 2013 Cochrane meta-analysis confirmed the primacy of high-dose, high-frequency interferon regimens over low-dose, low-frequency interferon regimens in reducing annualized relapse rates.1,4-8
Two comparative studies, REGARD and BEYOND, demonstrated no significant difference in annualized relapse rates in patients with RRMS who were treated with either high-dose, high-frequency interferons or with glatiramer acetate. However, preliminary evidence from the REGARD study indicated that brain volume loss was significantly slower in patients treated with glatiramer acetate versus patients treated with high-dose, high-frequency interferon beta-1a.6,8
Comparative Data with Oral Agents
Unlike the older agents, newer oral agents for RRMS have been approved with pivotal trial data that include their direct comparison with the older injectable agents. The TRANSFORMS trial compared interferon beta-1a with oral fingolimod, and the pivotal CONFIRM trial compared glatiramer acetate with oral DMF.2,9
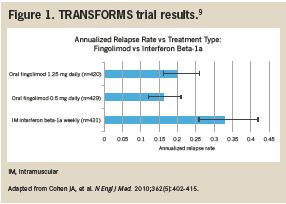
Data from the TRANSFORMS study showed the superiority of fingolimod to a low-dose, low-frequency interferon. This result came as no surprise considering that low-dose, low-frequency interferon was found inferior to high-dose, high-frequency interferon in 3 previous comparative trials (INCOMIN, EVIDENCE, and Etemadifar 2006) and in a 2013 Cochrane meta-analysis. Unfortunately, TRANSFORMS does not clarify the comparative efficacy of fingolimod with respect to high-dose, high-frequency interferon therapy or glatiramer acetate (Figure 1).1
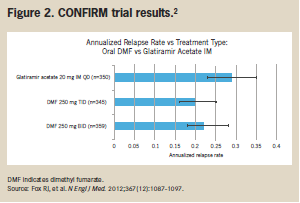
The CONFIRM trial compared DMF with glatiramer acetate in a head-to-head study. The results showed comparable reductions in annualized relapse rates with glatiramer acetate and with DMF. In addition, compared with placebo, reductions in annualized relapse rates were proportionally higher with DMF than with glatiramer acetate injection (44% with twice-daily DMF, 51% with 3-times-daily DMF, and 29% with glatiramer acetate (Figure 2).2
In summary, the availability of comparative pivotal trials with 2 oral agents (fingolimod and DMF) enables direct comparison of the tolerability, clinical efficacy, and expected reduction in relapse rates with these newer agents versus existing injectable agents. The CONFIRM trial and the TRANSFORMS trial offer patients with RRMS, and the health care professionals who care for them, more detailed information about the safety and efficacy offered by new agents for RRMS, with direct comparisons with older therapies.2,3
TRANSFORMS
The TRANSFORMS study was a 24-month,1027-patient extension trial that evaluated clinical results with patients transitioning from a low-dose, low-frequency interferon (weekly interferon beta-1a) to oral fingolimod in 1 of 2 doses (0.5 mg or 1.25 mg). All patients in the study had an initial Expanded Disability Status Scale (EDSS) score ≤5.5. Investigators monitored RRMS relapses, each patient’s EDSS every 3 weeks, and performed an MRI at baseline, month 3, and month 24.9 Annualized relapse rates were not significantly different with 0.5-mg or 1.25-mg fingolimod (16% and 20%, respectively). However, use of weekly interferon beta-1a was associated with a significantly higher annualized relapse rate (33%) than with either dose of fingolimod (P <.001).9
CONFIRM
The CONFIRM study was a 96-week, 1417-patient trial comparing clinical results with DMF 240 mg twice daily, DMF 240 mg 3 times daily, and a once-daily glatiramer acetate injection. All patients involved in the study had an initial Expanded Disability Status Scale (EDSS) score ≤5. Investigators monitored RRMS relapses and each patient’s EDSS every 12 weeks, and performed an MRI at baseline and at weeks 24, 48, and 96.2 Although all 3 treatments outperformed placebo, annualized relapse rates were lowest with DMF 240 mg twice daily (22%) and with DMF 240 mg 3 times daily (20%). In addition, annualized relapse rates were numerically, but not statistically, significantly higher with injectable glatiramer acetate (29%) than with either dose of DMF.2
References
1. Filippini G, Del Giovane C, Vacchi L, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2013;6:CD008933.
2. Fox RJ, Miller DH, Phillips JT, et al; for CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087-1097.
3. Cohen JA, Barkhof F, Comi G, et al; for TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402-415.
4. Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomized multicentre study (INCOMIN). Lancet. 2002;359(9316):1453-1460.
5. Schwid SR, Panitch HS. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther. 2007;29(9):2031-2048.
6. Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903-914.
7. Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing-remitting multiple sclerosis. Acta Neurol Scand. 2006;113(5):283-287.
8. O’Connor P, Filippi M, Arnason B, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8(10):889-897.
9. Khatri B, Barkhof F, Comi G, et al; for TRANSFORMS Study Group. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10(6):520-529.
10. National Multiple Sclerosis Society. Medications. http://www.nationalmssociety.org/Treating-MS/Medications. Accessed May 2014.
11. Koch-Henriksen N, Sorensen PS, Christensen T, et al. A randomized study of two interferon-beta treatments in relapsing-remitting multiple sclerosis. Neurology. 2006;66(7):1056-1060.
12. Kappos L, Radue EW, O’Connor P, et al; for FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387-401.
13. Radue EW, O’Connor P, Polman CH, et al; for FTY720 Research Evauating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) Study Group. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol. 2012;69(10):1259-1269.






