Study Compares Benefits of Treating Inverse Psoriasis with IL-17, IL-23 Inhibitors
This retrospective, systematic review highlighted the lack of studies specifically addressing the needs of patients with inverse psoriasis.
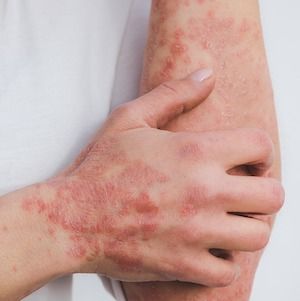
Individuals with inverse psoriasis (IP) do not respond as frequently to biologic treatments as individuals with psoriasis vulgaris, according to recent findings. While IL-17 inhibitors generally have faster onset than IL-23, these findings suggest that long-term anti-IL-23 therapies appear to be linked to better outcomes.1
These conclusions were the result of new research led by Luca Mastorino, from the University of Turin’s department of medical sciences’ section of dermatology in Italy. Mastorino et al. noted that there is still a lack of clear definition of the psoriasis subtype known as inverse psoriasis, in addition to the lack of a well-defined therapeutic strategy.2
“Data on other biological therapies are limited to a single case report for adalimumab and ustekinumab,” Mastorino and colleagues wrote. “A defined therapeutic strategy for a patient with IP is still unclear since few comparative studies exist on these drugs.”3
Background and Methods
The research team carried out a retrospective analysis of subjects with diagnoses of psoriasis who had also been treated with IL-23 or IL-17 inhibitor treatment, and the team followed their treatment path through the Dermatology Clinic of Turin University Hospital from January 2017 - September 2022. The team sought to evaluate these treatments’ efficacy among those with inverse psoriasis (IP) and to compare results with those who have vulgar psoriasis.
Furthermore, the investigators’ research was designed to characterize individuals with inverse psoriasis in relation to other psoriatic disease patients provided with these same types of medications. They sought to compare the effectiveness of IL-17 and IL-23 inhibitor therapies, specifically, within the subgroup of inverse psoriasis patients.
The research team classified subjects as having inverse psoriasis if they were shown to have disease involvement on specific regions such as their inguinal, axillary, breast folds, submammary lines, intergluteal fold, antecubital and popliteal pits, and perianal area. Psoriasis vulgaris patients with concurrent intertriginous involvement were determined to be under the vulgar psoriasis definition. Those whose medical records were inaccessible were not included in their research.
The investigators assessed effectiveness through the Psoriasis Area and Severity Index (PASI), uniformly implemented in the clinic with retrievable values for their evaluation. They looked at their severity of disease, epidemiological data, cardiovascular comorbidities, body mass index (BMI), diabetes status, and prior treatments using descriptive statistics.
When looking at the categorical variables, the research team calculated patient numbers as well as percentage proportions. Meanwhile, mean and standard deviation (SD) were utilized for continuous variables.
Findings
The investigators reported that individuals with inverse psoriasis were mostly female and more commonly treated with IL-17 inhibitors, experiencing a higher drug discontinuation rate and subsequent treatment alterations. Specifically, 32.1% of those with inverse psoriasis swapping treatments versus 18.1% of those with psoriasis vulgaris (P = .002).
Those with inverse psoriasis, over a period of time, were shown to have a slower progression to achievement of PASI100 and PASI90 versus people with vulgar psoriasis. The inverse psoriasis arm of the team’s research had increased joint involvement among individuals given anti-IL-17 therapies. These subjects were noted by the investigators as having a lower median age of onset (P = .011) versus subjects given anti-IL-23 treatments.
Those with inverse psoriasis on anti-IL-23 medications were reported to have begun treatment with a lower mean PASI and showed a slower rate of response versus those on anti-IL-17 treatments. Despite these findings, over extended periods, the research team did find that IL-23 inhibitors were progressively more impactful than IL-17.
“The limitations of our study are those inherent in its retrospective and real-life nature,” they wrote. “The comparison between patients with specific intertriginous involvement and those with psoriasis vulgaris is rendered weak by the significant difference in sample size…Similar considerations can be made for the imbalance between IL-17 and IL-23 inhibitors in the treatment of the IP population.”
References
- Mastorino L, Dapavo P, Ribero S, et al. Clinical characteristics and response to biological therapies for inverse psoriasis: a real-life comparison between the therapeutic effects of anti-IL-23 and anti-IL-17 agents. Int J Dermatol. 2024 May 15. doi: 10.1111/ijd.17252. Epub ahead of print. PMID: 38751026.
- Walsh JA, Jones H, Mallbris L, Duffin KC, Krueger GG, Clegg DO, et al. The physician global assessment and body surface area composite tool is a simple alternative to the Psoriasis Area and Severity Index for assessment of psoriasis: post hoc analysis from PRISTINE and PRESTA. Psoriasis. 2018; 8: 65–74. https://doi.org/10.2147/PTT.S169333.
- Hong JJ, Mosca ML, Hadeler EK, Brownstone ND, Bhutani T, Liao WJ. Genital and inverse/intertriginous psoriasis: an updated review of therapies and recommendations for practical management. Dermatol Ther. 2021; 11(3): 833–844.