Publication
Article
CYP2C19 Genotype, Clopidogrel Metabolism, Platelet Function, and Cardiovascular Events

Hitinder S. Gurm, MD
Review
Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and metaanalysis. JAMA. 2011;306:2704-2714.
Clopidogrel, a platelet adenosine diphosphate (ADP)-receptor an-tagonist, is a commonly prescribed medication that is used by over 40 million patients worldwide. Its use is nearly ubiquitous in patients with acute coronary syndromes or those undergoing coronary stenting. Clopidogrel is a pro-drug that requires oxidation by the hepatic cytochrome P450 (CYP450) to generate an active metabolite. This active metabolite forms a disulfide bridge with the 2 extracellular cysteine residues located on the ADP P2Y12 receptor expressed on the platelet surface, and causes an irreversible blockade of ADP binding for the platelet’s life span.1
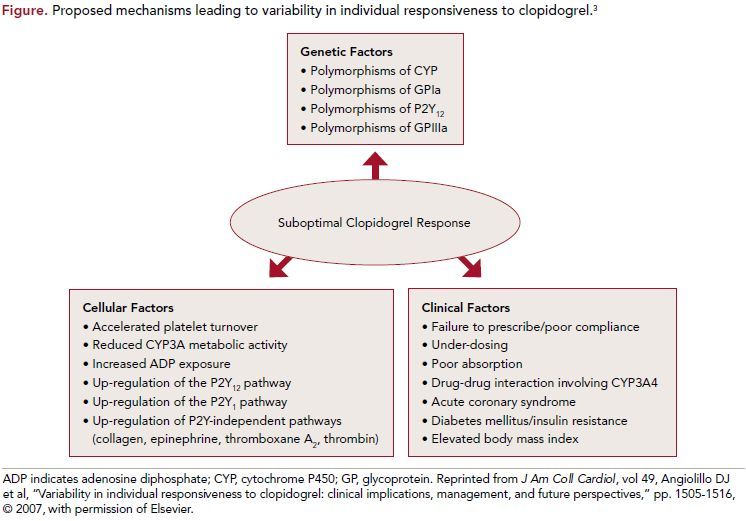
A standard dose of clopidogrel will achieve incomplete P2Y12 antagonism, which translates into approximately 50% inhibition of ADP-induced platelet aggregation. Various studies have demonstrated that somewhere between 4% and 30% of patients do not achieve adequate inhibition of platelet aggregation in response to clopidogrel and are assumed to be platelet resistant.2 The causes of platelet resistance vary and are outlined in the Figure.3
One of the mechanisms that has been invoked as a cause for clopidogrel resistance is inadequate conversion of clopidogrel to its pro-drug due to genetic variations in CYP2C19. This hypothesis has been tested in multiple studies but the results have been mixed. This issue gained increased importance after the FDA issued a black box warning on March 12, 2010, proposing that genotyping for CYP2C19 can be used to detect poor metabolizers of clopidogrel, and suggesting that alternative treatment or treatment strategies be considered in such patients.
The FDA warning was followed by a consensus statement from the American College of Cardiology and American Heart Association, which concluded
that there was insufficient evidence to support use of genotyping for guiding clopidogrel therapy.4 These opposing recommendations have caused confusion
about the clinical utility of pharmacogenomic guidance for tailoring clopidogrel therapy.
Holmes and colleagues performed a systematic review and meta-analysis to evaluate the association of CYP2C19 genotype with response to clopidogrel and the resulting clinical implications.5
Study Details
The authors performed a meta-analysis that included all studies that reported clopidogrel metabolism, platelet reactivity, or clinically relevant outcomes (cardiovascular disease [CVD] events and bleeding), and information on CYP2C19 genotype. The final analysis included 32 studies that enrolled 42,016 patients in whom there were 3545 CVD events, 579 stent thromboses, and 1413 bleeding events. Six of the included studies were randomized trials and the remaining 26 reported individuals exposed to clopidogrel (“treatment-only” design). Overall, these studies evaluated 13 of the 28 known CYP2C19 variant alleles. In treatment-only analysis, individuals
with 1 or more CYP2C19 alleles associated with lower enzyme activity had lower levels of active clopidogrel metabolites, less platelet inhibition, lower
risk of bleeding (RR, 0.84; 95% CI, 0.75-0.94; absolute risk reduction of 5 to 8 events per 1000 individuals), and higher risk of CVD events (RR, 1.18; 95% CI, 1.09-1.28; absolute risk increase of 8 to 12 events per 1000 individuals). Evidence of small-study bias was detected, and when the analyses were restricted to studies with 200 or
more events, the reduction in CVD events was not significant (RR, 0.97; 95% CI, 0.86-1.09).
Strong association was seen with respect to stent thrombosis and lossof- function alleles (RR, 1.75; 95% CI, 1.50-2.03), a finding that was also present when the analysis was restricted to studies with more than 100 events (RR, 1.54, 95% CI, 1.26-1.88). Overall, lossof- function alleles were associated with an absolute increase of 14 stent thrombosis events per 1000 individuals. In the placebo-controlled randomized trials, CYP2C19 genotype was not associated with modification of the effect of
clopidogrel on CVD events or bleeding. The relative risk of major CVD events in patients treated with clopidogrel compared with placebo was 0.78 (95% CI, 0.69-0.89) in genotype category *1 or *17 and the relative risk in genotype category *2 or *3 (slow metabolizers) was 0.87 (95% CI, 0.70-1.09). The authors concluded that while there was an association between the CYP2C19 genotype and clopidogrel responsiveness, there was no evidence to support an association of genotype with cardiovascular
events.
References
1. Pereillo JM, Maftouh M, Andrieu A, et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos. 2002;30:1288-1295.
2. Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol. 2005;45:1157-1164.
3. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505-1516.
4. Holmes DR, Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American
College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular
Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56:321-341.
5. Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review
and meta-analysis. JAMA. 2011;306:2704- 2714.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH,et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
8. Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097-1105.
COMMENTARY
Clopidogrel Responsiveness and CYP2C19 Genotype
The authors evaluated the totality of evidence linking CYP2C19 with clinical outcome in patients treated with clopidogrel and demonstrated a lack of association between alleles associated with poor metabolism of clopidogrel and cardiovascular events. The authors did find a decrease in platelet inhibition and a reduction in bleeding with loss-offunction alleles, suggesting that poor metabolism is associated with a reduction in the anti-platelet effect of the drug. This did not, however, translate into a
difference in cardiac events or bleeding in adequately powered studies. A strong association between stent thrombosis and loss-of-function alleles was noted, suggesting that this may be one population where genotyping may have a role.
Stent thrombosis is a rare but often devastating complication that appears to be reduced with more potent platelet inhibition. Both prasugrel and ticagrelor have
demonstrated dramatic reduction in stent thrombosis but at the cost of increased risk of bleeding.6,7 The choice of clopidogrel versus prasugrel or ticagrelor is going to become even more relevant in the near future once generic clopidogrel becomes available and the cost difference becomes a major factor influencing the
choice of the anti-platelet therapy.
There are many reasons, however, why pharmacogenomics may not be the easy answer to this clinical dilemma. The bioavailability of clopidogrel is influenced
by many factors other than CYP2C19, and reliance on CYP2C19 alone might induce a false sense of security or concern depending on the test results. Furthermore, it is not clear what level of platelet inhibition is ideal and use of functional testing of platelet inhibition to guide pharmacotherapy has not influenced clinical
outcomes when tested in a randomized fashion in
the GRAVITAS trial.8
For now, the decision to use prasugrel or ticagrelor instead of clopidogrel in a patient undergoing coronary stenting should be done with a careful assessment
of the patient’s risk of bleeding versus the risk of stent thrombosis based on anatomy and clinical factors. As this meta-analysis suggests, it may be premature to use
genomic information to tailor clopidogrel therapy.
The idea of personalized genomic-based anti-platelet therapy is seductive, but should be subject to the same rigorous proof of clinical efficacy that is expected of any new therapy or agent. Failure to do so will invariably lead to unnecessary and expensive testing and may end up misleading rather than correctly guiding therapy.
About the Author
Hitinder S. Gurm, MD , is associate professor, director of inpatient cardiology services, and director of carotid interventions in cardiovascular medicine in the Division of Cardiovascular Medicine at the University of Michigan Cardiovascular Center in Ann Arbor. He received his MD from Christian Medical College in Ludhiana, India, and completed his residency and fellowships at Cleveland Clinic Foundation. Dr Gurm’s research interests include outcomes research and quality improvement in percutaneous coronary and peripheral interventions and endovascular therapy of carotid disease. He is the principal investigator of the Blue Cross/Blue Shield of Michigan Cardiovascular Collaborative (BMC2), a statewide registry focused on improving safety and appropriateness of PCI across all hospitals in the state of Michigan.
