Publication
Article
Impact of Dietary Chocolate on Patients With Heart Failure

Jimena A. Blandon, MD
Review

Flammer JA, Sudano I, Wolfrum M, et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure [published online ahead of print December 15, 2011]. Eur Heart J. doi:10.1093/eurheartj/ehj448.
Heart failure (HF) treatment has seen a dramatic change in the last 20 years or so. Several medications have been shown to decrease mortality in these patients, targeting the renin-angiotensin-aldosterone system (RAAS) and the sympathetic system. However, there is still room for improvement because median survival
time among HF patients remains lower than among patients with some types of cancers.1
An increased risk of hospitalizations and death for HF patients has been associated with impaired endothelium-dependent vasodilation.2 An earlier study by Mostofsky and colleagues suggested that moderate habitual chocolate intake (ie, consuming 1 to 2 portions of chocolate per week) was associated with a lower rate of HF hospitalization or death in middle-aged and elderly women, but this effect was not seen if the amount of chocolate consumed was ≥1 serving per day.3
Possible mechanisms for endothelial dysfunction in HF include, but are not limited to, alterations in enzyme nitric oxide synthase (eNOS), activation of RAAS, and inactivation of NO by superoxide anions.4 Endothelial malfunction, extensively present in patients with HF, was the subject of a recent randomized controlled trial using flavanol- rich chocolate (FRC) versus cocoaliquor- free control chocolate (CC) by Flammer and colleagues.5 The study demonstrated an acute (at 2 hours) and long-term (at 4 weeks) beneficial effect of FRC on endothelial-dependent vasodilatation and an acute decrease in platelet adhesion.
Study Details
The authors assessed whether eating chocolate would improve cardiovascular function in patients with HF. They enrolled 22 patients with stable HF (NYHA ≥II) in a double-blind, randomized placebo-controlled trial, who were assigned to eat FRC or CC for up to 4 weeks. Patients were excluded if they had diabetes mellitus, hypotension, blood pressure >160/100 mm Hg, obesity, gastric ulcer, elevated aminotransferases or creatinine, or were smokers, among others conditions.
The study used FRC and CC chocolate bars prepared by Nestlé; both were wrapped identically to avoid potential bias for patients or examiners. The FRC bars contained 10.5 g of sugar, 17.9 g of fat per 40-g serving, with 70% cocoa content; CC bars contained a similar amount of carbohydrate and fat. Patients ingested 40 g of this product in 10 minutes, after at least 8 hours of fasting and 24 hours without flavanol- rich products; 2 hours later patients had a primary set of tests of endothelial and baroreceptor function and platelet adhesion measurements. They were then instructed to ingest 1 bar twice a day and continue their daily diets for 4 weeks, and testing was repeated at weeks 2 and 4.
The primary end point was endothelial function. The investigators assessed endothelial-dependent dilation by measuring flow-mediated dilatation (FMD) of the brachial artery after a 5-minute period with a wrist cuff inflated at 220 mm Hg. Endothelial-independent dilation was measured with continuous arterial measurement after sublingual administration of 0.4 mg of glyceryl trinitrate. There were 3 secondary end points: (a) baroreceptor function, measured using blood pressure (BP) and
heart rate (HR); (b) platelet adhesion, assessed with a shear stress-dependent
model; and c) oxidative stress parameters, using the total radical-reducing antioxidant potential (TRAP) method. In addition, plasma levels of flavanols (catechin and epicatechin) were measured. Two of the 22 patients were not included in the final analysis: 1 dropped out for personal reasons and the other had a ventricular fibrillation episode considered not related to treatment. The study and control groups were similar in baseline characteristics and remained stable during the study as per
unchanged levels of pro-B-natriuretic peptide; patients did not gain weight over the 4-week course of chocolate ingestion.
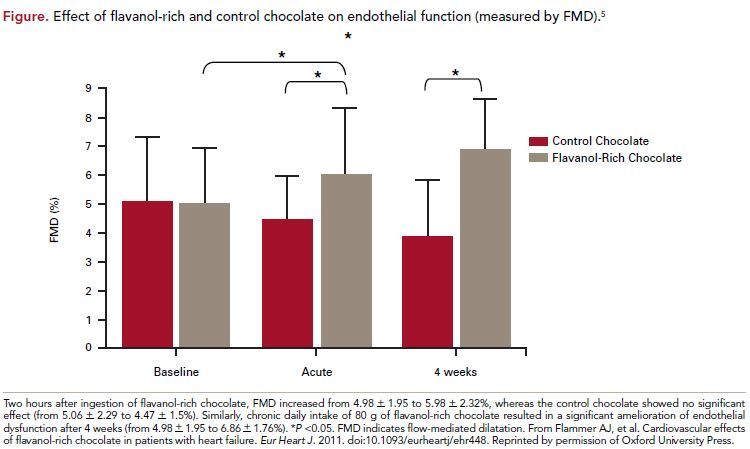
With respect to the primary end point, FMD significantly improved from 4.98 ± 1.95 to 5.98 ± 2.32% (P = 0.045 and 0.02 for between-group changes) 2 hours after intake of FRC. After 4 weeks of daily intake this difference was even bigger (6.86 ± 1.76%) for a P = 0.03 and 0.004 between groups. However, endothelial-independent vasodilatation did not improve. The secondary end point of platelet adhesion significantly decreased from 3.9 ± 1.3 to 3.0 ± 1.3% (P = 0.03 and 0.05 for between groups) 2 hours after FRC, but this effect was not sustained at 2 and 4 weeks. Blood pressure and heart rate did not change in either group. Cocoaliquor-free CC had no effect, either on endothelial function or on platelet function, but decreased insulin sensitivity, an effect not observed with FRC. There were no changes in oxidative stress
markers with either treatment group acutely or at 4 weeks. On the other hand, an increase in epicatechin at 2 hours was noted in patients who took FRC compared with patients that had CC (from below detection limit to 0.69 ± 0.28 μM, P <0.001 for intra- and between-group changes); this effect did not persist at 4 weeks (Figure).
Overall, treatment with FRC did significantly improve the primary end point compared with CC, and platelet adhesion was decreased with acute ingestion of chocolate, consistent with an increase in flavanol plasma levels. However, this effect was not present at 4 weeks’ evaluation.
REFERENCES
1. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:1343-1382.
2. Katz SD, Hryniewicz K, Hriljac I, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310-314.
3. Mostofsky E, Levitan EB, Wolk A, Mittleman MA. Chocolate intake and incidence of heart failure: a population-based, prospective study of middle-aged and elderly women. Circ Heart Fail. 2010;3:612-616.
4. Helmut D, Hornig B, Landmesser U. Alterations in the peripheral circulation in heart failure. In: Mann DL. Heart Failure: A Companion to Braunwald’s Heart Disease.
2nd ed. St. Louis, MO: Elsevier-Saunders; 2011:279-290.
5. Flammer AJ, Sudano I, Wolfrum M, et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure [published online ahead of print December 15, 2011]. Eur Heart J. doi:10.1093/eurheartj/ehj448.
6. Grassi D, Necozione S, Lippi C, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398-405.
7. Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr. 2008;88:58-63.
8. Hollenberg NK, Fisher ND. Is it the dark in dark chocolate? Circulation. 2007;116:2360-2362.
COMMENTARY
Effects of Flavanol-Rich Chocolate on Endothelial Malfunction
This study sheds additional light on the beneficial effects of flavanol-rich cocoa on cardiovascular health. It has already been demonstrated that moderate chocolate ingestion can reduce blood pressure and insulin resistance, and improve endothelium-dependent vasodilatation in hypertensive patients.6
The current study was a randomized controlled trial with robust design to assess proposed end points. It is also the first study to use chocolate to treat HF patients on top of standard medical management, and it did show improvement in physiological variables without deterioration of patients’ clinical status over 1 month.
Some caveats need to be taken into consideration. The sample size was very small, with final analysis based on only 20 patients, and did not include diabetics or smokers, which would be more representative of a real-world population. The study lasted only 4 weeks, probably good enough for the end points, but not long enough to draw conclusions about long-term effects on morbidity or mortality. That will take at least several months to years. Many other therapies directed to altered HF physiology have been proved beneficial in short-term trials but have not proved useful in long-term studies, such as inotropes or neseritide.1
The caloric intake derived from eating 2 bars of FRC daily was 400 calories with 36 g of fat, and even though the patients’ weight did not change in 1 month, a longer follow-up will allow us to have a more complete picture, as it is a concern that the additional calories and fat would encourage the development of fatty liver, diabetes,
and increased insulin resistance. In addition, this design did not control for usual daily dietary intake, an aspect that is very important in any study involving nutritional manipulation.
It is hypothesized that the benefits of this treatment rely on its high amount of polyphenols, for example, procyanidins7; however, levels of polyphenols were only increased 2 hours after ingestion but not at the end of 4 weeks, paralleling the acute, but not chronic, decrease in platelet adhesion. This suggests that there is probably an
alternative beneficial mechanism that would explain the long-term (4 weeks) improvement in FMD.
It will be interesting to see a study like this undertaken with a larger number of patients, longer-term follow-up (years, not weeks), and assessments of mortality and morbidity parameters (hard events like death, hospitalizations for HF, improvement in quality of life, and NYHA class) in addition to just hemodynamic or physiologic
surrogate variables. We need a more definitive therapeutic arsenal to offer to our HF patients, but we have to be sure it’s not going to cause harm in the long
run. Treatment with chocolate could be an alternative for specific patients with a stable clinical picture, without risk for obesity, diabetes, or gastroesophageal reflux disease, and patients who need a good nutritional supplement (but cardiac cachexia would mean an advanced state of HF that will not likely improve by eating a daily dose of chocolate).
One should not forget that chocolate is quite high in calories and in fat. It should be noted that manufacturers need to put a label on each package of chocolate that describes the flavanol content of the chocolate, since the term “dark” may be misleading; the color of the chocolate may have nothing to do with flavanol content, and many manufacturers use processing techniques that destroy flavanols, even when the product is labeled dark.8
Until then, we should be paying attention to future reports on this exciting and gastronomically pleasant treatment, and hope for some excellent and yummy chocolate flavors for our patients. We will be watching with anticipation!
About the Author
Jimena A. Blandon, MD, is a second-year resident instructor in the Department of Internal Medicine at Texas Tech University Health Sciences Center in El Paso. She received her MD at Universidad del Valle, in Cali, Colombia. Dr Blandon is currently involved in a study of the prevalence and predictors of contrastinduced nephropathy in a predominantly Hispanic population among patients undergoing angiography at the University Medical Center at El Paso, Texas.






